An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Comprehensive Review on Alzheimer’s Disease: Causes and Treatment
Zeinab breijyeh, rafik karaman.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2020 Nov 5; Accepted 2020 Dec 6; Collection date 2020 Dec.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/ ).
Alzheimer’s disease (AD) is a disorder that causes degeneration of the cells in the brain and it is the main cause of dementia, which is characterized by a decline in thinking and independence in personal daily activities. AD is considered a multifactorial disease: two main hypotheses were proposed as a cause for AD, cholinergic and amyloid hypotheses. Additionally, several risk factors such as increasing age, genetic factors, head injuries, vascular diseases, infections, and environmental factors play a role in the disease. Currently, there are only two classes of approved drugs to treat AD, including inhibitors to cholinesterase enzyme and antagonists to N -methyl d -aspartate (NMDA), which are effective only in treating the symptoms of AD, but do not cure or prevent the disease. Nowadays, the research is focusing on understanding AD pathology by targeting several mechanisms, such as abnormal tau protein metabolism, β-amyloid, inflammatory response, and cholinergic and free radical damage, aiming to develop successful treatments that are capable of stopping or modifying the course of AD. This review discusses currently available drugs and future theories for the development of new therapies for AD, such as disease-modifying therapeutics (DMT), chaperones, and natural compounds.
Keywords: Alzheimer’s disease, neurodegeneration, β-amyloid peptide, tau protein, risk factors, disease-modifying therapy, chaperons, heat shock proteins
1. Introduction
Alzheimer’s disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles ( Figure 1 ) as a result of amyloid-beta peptide’s (Aβ) accumulation in the most affected area of the brain, the medial temporal lobe and neocortical structures [ 1 ]. Alois Alzheimer noticed a presence of amyloid plaques and a massive loss of neurons while examining the brain of his first patient that suffered from memory loss and change of personality before dying and described the condition as a serious disease of the cerebral cortex. Emil Kraepelin named this medical condition Alzheimer’s disease for the first time in his 8th edition psychiatry handbook [ 2 , 3 ]. Progressive loss of cognitive functions can be caused by cerebral disorder like Alzheimer’s disease (AD) or other factors such as intoxications, infections, abnormality in the pulmonary and circulatory systems, which causes a reduction in the oxygen supply to the brain, nutritional deficiency, vitamin B12 deficiency, tumors, and others [ 4 , 5 ].
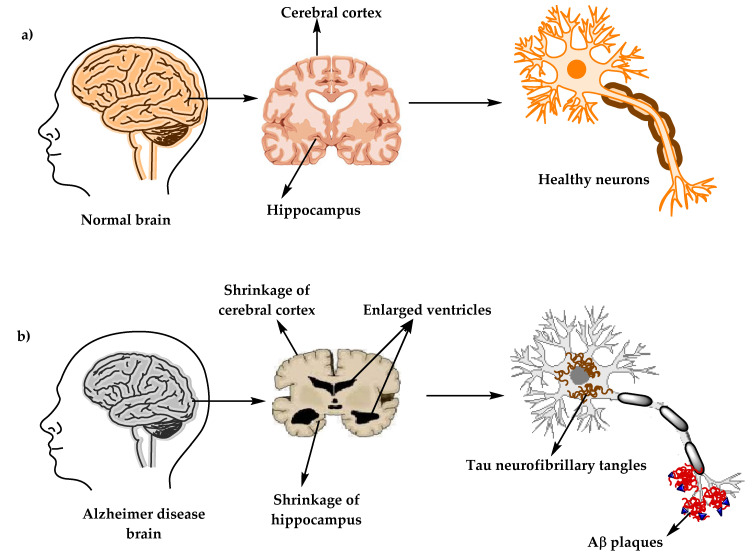
The physiological structure of the brain and neurons in ( a ) healthy brain and ( b ) Alzheimer’s disease (AD) brain.
At present, there are around 50 million AD patients worldwide and this number is projected to double every 5 years and will increase to reach 152 million by 2050. AD burden affects individuals, their families, and the economy, with estimated global costs of US$1 trillion annually. At present, there is no cure for Alzheimer’s disease, although there are available treatments that just improve the symptoms [ 6 , 7 ]. The purpose of this review is to give a brief description about AD diagnosis, pathology, causes, and current treatments, and to highlight the recent development of compounds that could prevent or treat AD by targeting several pathogenic mechanisms, such as Aβ and tau aggregation, and misfolding, inflammation, oxidative damage, and others.
2. Alzheimer’s Disease Diagnostic Criteria
A patient suspected to have AD should undergo several tests, including neurological examination, magnetic resonance imaging (MRI) for neurons, laboratory examinations such as vitamin B12, and other tests besides the medical and family history of the patients [ 8 ]. Vitamin (vit.) B12 deficiency has been long known for its association with neurologic problems and increasing risks of AD, according to some studies. A special marker of vit. B12 deficiency is elevated homocysteine levels, which can cause brain damage by oxidative stress, increasing calcium influx and apoptosis. Diagnoses of vit. B12 deficiency can be done by measuring serum vit. B12 level alongside complete blood count and serum homocysteine levels tests [ 9 , 10 ].
In 1984, The National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) formed a work group (NINCDS-ADRDA) to establish a clinical diagnostic’s criteria for Alzheimer’s disease. This criteria includes: (1) probable Alzheimer’s disease, which can be diagnosed by dementia that is confirmed by neuropsychological tests, progressive memory loss, impaired daily-life activity, and other symptoms like aphasia (impairment of a language), apraxia (a motor skills disorder), and agnosia (a loss of perception). All of these symptoms can start from age 40–90, with the absence of any systemic or brain diseases, (2) possible Alzheimer’s disease can be applied in the absence of neurologic, psychiatric disorders, and the presence of another illness like systemic or brain disorder, but they are not the primary cause of dementia, and (3) definite Alzheimer’s disease, that is confirmed by histopathologic confirmation obtained from a biopsy or autopsy [ 11 , 12 ].
In 2011, The National Institute on Aging—Alzheimer’s Association made several changes and updated the 1984 NINCDS-ADRDA criteria for higher specificity and sensitivity in the diagnosis of Alzheimer’s disease. The newly proposed criteria include probable and possible AD dementia for the use in clinical settings and probable or possible AD dementia with pathophysiological evidence for research purposes, in addition to clinical biomarkers. There are two categories of Alzheimer’s disease biomarkers: (a) markers of brain amyloid such as positron emission tomography (PET) and cerebrospinal fluid (CSF), and (b) markers of neuronal injury like cerebrospinal fluid tau, fluorodeoxyglucose (FDG) for metabolic activity, and magnetic resonance imaging (MRI) for atrophy measurement [ 13 , 14 , 15 ].
3. Alzheimer’s Disease’s Neuropathology
There are two types of neuropathological changes in AD which provide evidence about disease progress and symptoms and include: (1) positive lesions (due to accumulation), which are characterized by the accumulation of neurofibrillary tangles, amyloid plaques, dystrophic neurites, neuropil threads, and other deposits found in the brains of AD patients. In addition to (2) negative lesions (due to losses), that are characterized by large atrophy due to a neural, neuropil, and synaptic loss. Besides, other factors can cause neurodegeneration such as neuroinflammation, oxidative stress, and injury of cholinergic neurons [ 16 , 17 , 18 ].
3.1. Senile Plaques (SP)
The senile plaques are extracellular deposits of beta-amyloid protein (Aβ) with different morphological forms, including neuritic, diffuse, dense-cored, or classic and compact type plaques. Proteolytic cleavage enzymes such as β-secretase and γ-secretase are responsible for the biosynthesis of Aβ deposits from the transmembrane amyloid precursor protein (APP) [ 19 , 20 , 21 ]. These enzymes cleave APP into several amino acid fragments: 43, 45, 46, 48, 49, and 51 amino acids, which reach the final forms Aβ40 and Aβ42. There are several types of Aβ monomers, including large and insoluble amyloid fibrils which can accumulate to form amyloid plaques and soluble oligomers that can spread throughout the brain. Aβ plays a major role in neurotoxicity and neural function, therefore, accumulation of denser plaques in the hippocampus, amygdala, and cerebral cortex can cause stimulation of astrocytes and microglia, damage to axons, dendrites, and loss of synapses, in addition to cognitive impairments [ 21 , 22 , 23 ].
3.2. Neurofibrillary Tangles (NFTs)
NFT are abnormal filaments of the hyperphosphorylated tau protein that in some stages can be twisted around each other to form paired helical filament (PHF) and accumulate in neuralperikaryal cytoplasm, axons, and dendrites, which cause a loss of cytoskeletal microtubules and tubulin-associated proteins. The hyperphosphorylated tau protein is the major constituent of NFTs in the brains of AD patients, and its evolution can reflect NFTs morphological stages, which include: (1) pre-tangle phase, one type of NFT, where phosphorylated tau proteins are accumulated in the somatodendritic compartment without the formation of PHF, (2) mature NFTs, which are characterized by filament aggregation of tau protein with the displacement of the nucleus to the periphery part of the soma, and (3) the extracellular tangles, or the ghost NFTs stage, that results from a neuronal loss due to large amounts of filamentous tau protein with partial resistance to proteolysis [ 24 , 25 ].
3.3. Synaptic Loss
A synaptic damage in the neocortex and limbic system causes memory impairment and generally is observed at the early stages of AD. Synaptic loss mechanisms involve defects in axonal transport, mitochondrial damage, oxidative stress, and other processes that can contribute to small fractions, like the accumulation of Aβ and tau at the synaptic sites. These processes eventually lead to a loss of dendritic spines, pre-synaptic terminals, and axonal dystrophy [ 26 ]. Synaptic proteins serve as biomarkers for the detection of synapses loss, and severity, such as neurogranin, a postsynaptic neuronal protein, visinin-like protein-1 (VILIP-1), and synaptotagmin-1 [ 27 , 28 ].
4. The Stages of Alzheimer’s Disease
The clinical phases of Alzheimer’s disease can be classified into (1) pre-clinical or the pre-symptomatic stage, which can last for several years or more. This stage is characterized by mild memory loss and early pathological changes in cortex and hippocampus, with no functional impairment in the daily activities and absence of clinical signs and symptoms of AD [ 1 , 29 , 30 ]. (2) The mild or early stage of AD, where several symptoms start to appear in patients, such as a trouble in the daily life of the patient with a loss of concentration and memory, disorientation of place and time, a change in the mood, and a development of depression [ 30 , 31 ]. (3) Moderate AD stage, in which the disease spreads to cerebral cortex areas that results in an increased memory loss with trouble recognizing family and friends, a loss of impulse control, and difficulty in reading, writing, and speaking [ 30 ]. (4) Severe AD or late-stage, which involves the spread of the disease to the entire cortex area with a severe accumulation of neuritic plaques and neurofibrillary tangles, resulting in a progressive functional and cognitive impairment where the patients cannot recognize their family at all and may become bedridden with difficulties in swallowing and urination, and eventually leading to the patient’s death due to these complications [ 1 , 32 ].
5. Causes and Risk Factors of Alzheimer’s Disease
AD has been considered a multifactorial disease associated with several risk factors ( Figure 2 ) such as increasing age, genetic factors, head injuries, vascular diseases, infections, and environmental factors (heavy metals, trace metals, and others). The underlying cause of pathological changes in Alzheimer’s disease (Aβ, NFTs, and synaptic loss) is still unknown. Several hypotheses were proposed as a cause for AD but two of them are believed to be the main cause: some believe that an impairment in the cholinergic function is a critical risk factor for AD, while others suggest that alteration in amyloid β-protein production and processing is the main initiating factor. However, at present, there is no accepted theory for explaining the AD pathogenesis [ 33 , 34 ].
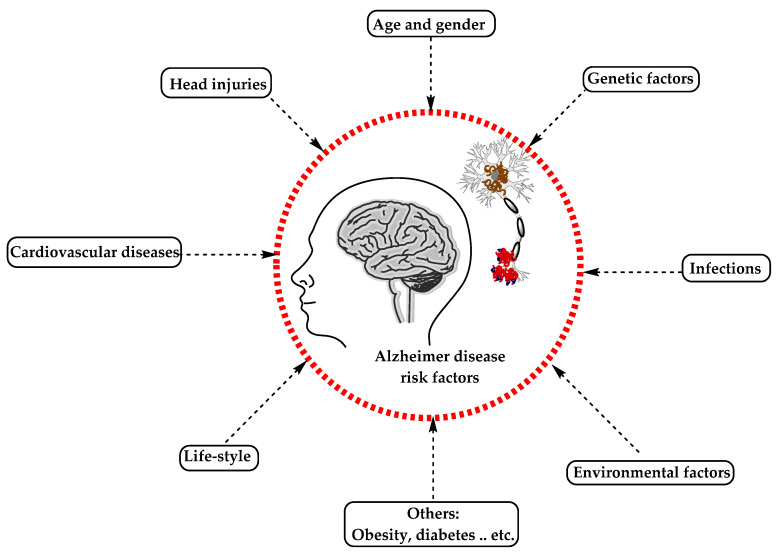
The risk factors for Alzheimer’s disease.
5.1. Alzheimer’s Disease Hypotheses
5.1.1. cholinergic hypothesis.
In the 1970s, neocortical and presynaptic cholinergic deficits were reported to be related to the enzyme choline acetyltransferase (ChAT), which is responsible for the synthesis of acetylcholine (ACh). Due to the essential role of ACh in cognitive function, a cholinergic hypothesis of AD was proposed. ACh is synthesized in the cytoplasm of cholinergic neurons from choline and acetyl-coenzyme A by the ChAT enzyme and transported to the synaptic vesicles by vesicular acetylcholine transporter (VAChT) ( Figure 3 ). In the brain, ACh is involved in several physiological processes such as memory, attention, sensory information, learning, and other critical functions. Degeneration of the cholinergic neurons was found to take place in AD and to cause alternation in cognitive function and memory loss. Β -amyloid is believed to affect cholinergic neurotransmission and to cause a reduction in the choline uptake and a release of ACh. Studies demonstrated that cholinergic synaptic loss and amyloid fibril formation are related to Aβ oligomers’ neurotoxicity and to interactions between AChE and Aβ peptide. Additional factors also contribute to the progression of AD, such as a reduction in nicotinic and muscarinic (M2) Ach receptors, located on presynaptic cholinergic terminals, and the deficit in excitatory amino acid (EAA) neurotransmission, where glutamate concentration and D-aspartate uptake are significantly reduced in many cortical areas in AD brains. This is in addition to the use of cholinergic receptor antagonists such as scopolamine, which was found to induce amnesia. This effect can be reversed by using compounds that activate acetylcholine formation [ 35 , 36 , 37 ].
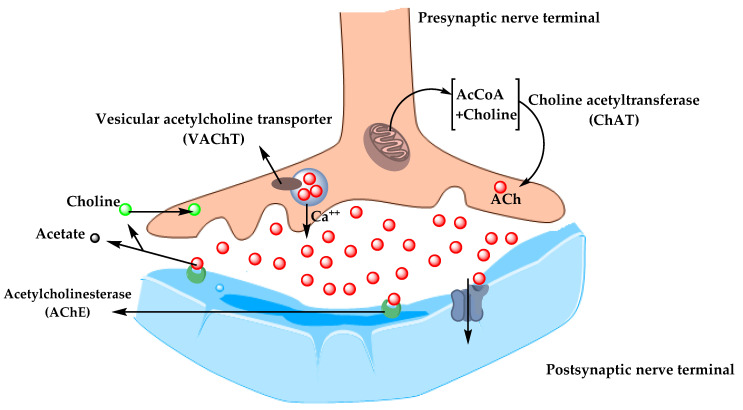
The pathway for the synthesis and transportation of acetylcholine between presynaptic and postsynaptic nerve terminals.
As a result, the cholinergic hypothesis is based on three concepts: reduced presynaptic cholinergic markers in the cerebral cortex, severe neurodegeneration of nucleus basalis of Meynert (NBM) in the basal forebrain, which is the source of cortical cholinergic innervation, and the role of cholinergic antagonists in memory decline compared to the agonists, which have the opposite effect [ 38 ].
5.1.2. Amyloid Hypothesis
For decades, it was recognized that abnormal deposition of β-sheets in the central nervous system has a strong correlation with dementia, which led to the concept of the amyloid hypothesis. However, it was found that the amyloid plaques (AP) also deposit in normal healthy brains with aging, which raised the question of whether AP deposition is responsible for AD onset or not? Therefore, in the recent years, alternative hypotheses were proposed for the non-inherited form of AD (NIAD), but at present, the amyloid hypothesis remains the most accepted pathological mechanism for inherited AD (IAD). The amyloid hypothesis suggests that the degradation of Aβ, derived from APP by β- and γ-secretase, is decreased by age or pathological conditions, which leads to the accumulation of Aβ peptides (Aβ40 and Aβ42). Increasing the ratio of Aβ42/Aβ40 induces Aβ amyloid fibril formation, resulting in neurotoxicity and tau pathology induction, and consequently, leading to neuronal cell death and neurodegeneration. AD risk factors and mutations of several genes like APP, PSEN1, and PSEN2 were found to affect Aβ catabolism and anabolism, which rapidly cause an accumulation of Aβ and fast progression of neurodegeneration [ 39 , 40 , 41 ].
5.2. Alzheimer’s Disease Risk Factors
5.2.1. aging.
The most important risk factor in AD is aging. Younger individuals rarely have this disease, and most AD cases have a late onset that starts after 65 years of age [ 42 ]. Aging is a complex and irreversible process that occurs through multiple organs and cell systems with a reduction in the brain volume and weight, a loss of synapses, and ventricles’ enlargement in specific areas accompanied by SP deposition and NFT. Moreover, several conditions might emerge during aging such as glucose hypometabolism, cholesterol dyshomeostasis, mitochondria dysfunction, depression, and cognitive decline. These changes also appear in normal aging, which makes it difficult to distinguish the cases in early AD [ 43 , 44 ]. AD can be divided based on age of onset into early-onset AD (EOAD), the rare form with around 1–6% of cases, in which most of them are familial AD characterized by having more than one member in more than one generation with AD, and ranges from 30–60 or 65 years. The second type is the late-onset AD (LOAD), which is more common with age of onset above 65 years. Both types may occur in people who have a family with a positive history of AD and families with a late-onset disease [ 45 ].
5.2.2. Genetics
Genetic factors were discovered over the years and were found to play a major role in the development of AD. 70% of the AD cases were related to genetic factors: most cases of EOAD are inherited in an autosomal dominant pattern and mutations in the dominant genes such as Amyloid precursor protein (APP) , Presenilin-1 (PSEN-1), Presenilin-2 (PSEN-2) , and apolipoprotein E (ApoE) are associated with AD [ 46 , 47 ].
Herein, we discuss the strong genetic risk factors in AD.
Amyloid Precursor Protein (APP)
APP is a type I transmembrane protein cleaved by α-, β-, and γ-secretase to release Aβ and other proteins and is encoded by the APP gene on chromosome 21. Thirty mutations have been found in the APP gene in which twenty-five of them are related to AD and cause an accumulation of Aβ with elevated amounts. Meanwhile, there is one protective mutation, A673T, which protects against AD by decreasing Aβ, Aβ40, and Aβ42 secretion [ 48 , 49 ]. All mutations surround the secretase cleavage site, for example, the KM670/671NL mutation in mouse models has shown an increasing level of amyloid plaques in the hippocampus and cortex with no NFTs. A673V, D678H, D678N, E682K, and K687N mutations have shown cortical atrophy, whereas E682K has shown hippocampal atrophy. Neuropathological reports for the A673V mutation demonstrated a presence of NFTs and Aβ, activation of microglia and astrocytes, and neuronal loss, compared to the rest of the mentioned mutations, which show no change in the intracellular Aβ according to neuropathological reports [ 48 , 50 ]. Other mutations such as T714I, V715A, V715M, V717I, V717L, L723P, K724N, and I716V affect the γ-secretase cleavage site and cause an increase in the Aβ42/Aβ40 ratio, while E693G, E693K, D694N, and A692G mutations affect the α-secretase cleavage site and cause polymorphic aggregates with the ability to disrupt bilayer integrity. Also, the E693delta is a deletion mutation that enhances the formation of synaptotoxic Aβ [ 51 , 52 ].
Presenilin-1 (PSEN-1) and Presenilin-2 (PSEN-2)
PSEN1 and PSEN2 genes are also the autosomal dominant form of EOAD located on chromosomes 14 and 1, respectively. PSEN-2 and PSEN-1 are homologous, with 67% similarity, with a difference in the N -terminus and the hydrophilic region. Mutation in PSEN1 gene is more common, with more than 200 mutations, while a rare form with less than 40 mutations was identified in the PSEN2 gene [ 53 , 54 ].
PSEN1 is a core protein that activates the γ-secretase complex and plays an important role in the production of Aβ from APP. Knockout studies of PSEN1 showed synaptic dysfunction and memory impairment in mice, which indicate its essential role in maintaining memory and neurons [ 51 ]. PSEN1 mutations are simple ones which include single amino acid substitution, and severe mutation can result from the substitutions of two amino acids [ 55 ]. Mutations in the PSEN1 gene increase the ratio of Aβ42/Aβ40 by decreasing Aβ40 levels. The results obtained by Sun et al. study demonstrated that C410Y or L435F mutations in PSEN1 knock-in mice increased the Aβ42/Aβ40 ratio due to a greater reduction in Aβ40 [ 56 ].
In contrast, PSEN-2 mutations are rare and play a minor role in Aβ production. Any mutation in PSEN-2 might have a severe effect on the Aβ 42/40 ratio, causing familial AD in the presence of normal PSEN-1 alleles. Some of the PSEN-2 mutations cause a significant increase in γ-secretase activity with an elevation in the Aβ-42 and Aβ 42/40 ratio level, such as N141I, T122P, M239V, and M239I, while others are rare polymorphisms and have no effect on Aβ-42, -40, and Aβ 42/40 ratio levels and are not considered as pathogenic mutations [ 53 , 57 ].
Apolipoprotein E (ApoE)
ApoE protein is a glycoprotein expressed highly in the liver and brain astrocytes and some microglia and serves as a receptor-mediated endocytosis ligand for lipoprotein particles like cholesterol, which is essential for myelin production and normal brain function. The ApoE gene located on chromosome 19 has three isoforms, ApoE2, ApoE3, and ApoE4, due to single-nucleotide polymorphisms (SNPs) which cause changes in the coding sequence. The ApoEε4 allele is a strong risk factor for both EOAD and LOAD compared to ApoEε2 and ApoEε3 alleles that are associated with a lower risk and protective effect, respectively [ 58 ]. ApoEε4 plays an important role in Aβ deposition as a senile plaque and causes cerebral amyloid angiopathy (CAA), which is known as a marker for AD [ 59 ]. ApoEε4 was also shown to be associated with vascular damage in the brain, which leads to AD pathogenesis [ 60 ].
ATP Binding Cassette Transporter A1 (ABCA1)
Adenosine triphosphate (ATP)-binding cassette transporter A1 (ABCA1) is part of a large ABC transporters family that regulate cholesterol efflux in the circulation, like apolipoproteins-AI (ApoAI), and into the brain, like ApoE. In addition, ABCA1 maintains the stability of ApoE lipidation and serves as a mediator for high-density lipoprotein (HDL) generation, which reflects its role in atherosclerosis and cardiovascular diseases. Studies on the AD mice model showed that ABCA1 deficiency increases amyloid plaques and eliminates the lipidation of ApoE [ 61 ]. In humans, a mutation in ABCA1 results in Tangier disease, which is characterized by low levels of high-density lipoprotein (HDL) and ApoAI in plasma, accumulation of cholesterol in tissues, and AD pathogenesis [ 62 ].
Clusterin Gene (CLU) and Bridging Integrator 1 ( BIN1 )
In contrast to PSEN1 , PSEN2 , and APP mutations, which result in familial or EOAD, clusterin ( CLU) and Bridging Integrator 1 ( BIN1 ) genes are novel risk factors for LOAD. In 2009, Genome-Wide Association Studies (GWAS) identified the CLU gene located on chromosome 8, which is upregulated in the cortex and hippocampus of AD brains, in addition to AD cerebrospinal fluid (CSF) and plasma, which make the CLU a promising biomarker for AD. The CLU may play a protective role by interacting with Aβ and promoting its clearance, or a neurotoxic role by reducing Aβ clearance. The Aβ ratio values determine whether the CLU role is neuroprotective or neurotoxic [ 63 ].
BIN1 is a Bin-Amphiphysin-Rvs (BAR) adaptor protein that is involved in the production of membrane curvature and other endocytosis cellular functions. BIN1 has several isoforms: some are found in the brain, where they interact with different proteins such as clathrin, synaptojanin, and amphiphysin 1, and others in which they regulate synaptic vesicle endocytosis. Recently, BIN1 was recognized as the second most important risk factor for LOAD after ApoE, where it plays a role in Aβ production and as a tau and NFT pathology modulator [ 64 , 65 ].
Evolutionarily Conserved Signaling Intermediate in Toll pathway (ECSIT)
A significant accumulation of Aβ in AD brains increases protein oxidation, which reflects the critical role of mitochondria in Aβ cytotoxicity and AD pathogenesis. Evolutionarily conserved signaling intermediate in Toll pathway (ECSIT) gene is located on chromosome 19 and is associated with increasing the risk of AD. ECSIT encodes the adapting protein that functions as a cytoplasmic and signaling protein and is responsible for stabilizing the mitochondrial respiratory complex. Moreover, the adaptor protein is involved in the activation of nuclear factor (NF)-κB, interferon regulatory factors (IRFs), and activating protein-1. Also, it is involved in coupling immune toll-like receptor (TLR), homeostatic bone morphogenetic pathway (BMP), and transforming growth factor-beta (TGF-b) pathways [ 66 , 67 ].
ECSIT interacts with mitochondrial proteins such as Lon protease homolog (LONP1) and glutaryl-CoA dehydrogenase (GCDH), which are involved in intra-mitochondrial proteolysis and redox signaling respectively, followed by interactions with AD seed nitric oxide synthase (NOS3). Moreover, studies have shown certain interactions of ECSIT with the AD genes ApoE , PSEN-1 , and PSEN-2 . These interactions support the role of ECSIT as a molecular link in oxidative stress, inflammation, and mitochondrial dysfunction in AD [ 66 , 68 ].
Estrogen Receptor Gene (ESR)
AD affects both women and men, but nearly two-thirds of AD cases are women. Several studies have shown that women with AD experience worse mental deterioration than men. Additionally, on the genetic level, some genes’ variation, like the ApoE4 allele, significantly increases AD risk in women compared to men. Other studies documented that AD risk in women is associated with the loss of ovarian hormones during menopause due to the fact that estrogen regulates several activities in the brain, such as neurotransmission, neural development, survival, protection against oxidative stress, reduction of Aβ peptide levels, and attenuation of tau hyperphosphorylation. The estrogen activity is mediated through estrogen receptors (ERs) (intracellular, transmembrane, and membrane-bound ERs). The two major subtypes of these receptors are ERα and Erβ, which are encoded by two distinct genes and are located on chromosome 6 and 14, respectively. ERα receptor is found in the hypothalamus and amygdala, whereas ERβ receptors are in the hippocampus and cortex. Single nucleotide polymorphisms (SNPs) in ERβ and ERα genes may affect exogenous estrogen in older women and influence cognitive aging. PvuII (rs9340799) and Xbal (rs223493) are examples of SNPs found in ERα and are associated with AD and cognitive impairment. Also, several SNPs in ERβ have been proven to increase the risk of AD in women [ 69 , 70 , 71 , 72 ].
Other Genes
Other genes’ polymorphism associated with increasing the risk of AD include vitamin D receptor (VDR) gene polymorphism, which affects the affinity of vitamin D to its receptor and may cause neurodegenerative diseases and neuronal damage [ 73 ]. Moreover, epigenetic factors like DNA methylation, histone, and chromatin modifications were demonstrated to be involved in AD [ 33 , 74 ].
5.2.3. Environmental Factors
Aging and genetic risk factors cannot explain all cases of AD. Environmental risk factors including air pollution, diet, metals, infections, and many others may induce oxidative stress and inflammation and increase the risk for developing AD. Herein, we report the most important environmental factors and their relationships with AD [ 75 , 76 ].
Air Pollution
The air pollution is characterized by modifying the nature of the atmosphere through the introduction of chemical, physical, or biological pollutants. It is associated with respiratory and cardiovascular diseases and recently, its association with AD was documented. Six air pollutants have been defined by National Ambient Air Quality Standards (NAAQSs) in the USA as a threat to human health, including ozone (O 3 ), nitrogen oxides (NO x ), carbon monoxide (CO), particulate matter (PM), sulfur dioxide (SO 2 ), and lead. Studies on animals and cellular models have shown that an exposure to high levels of air pollution can result in a damage to the olfactory mucosa and bulb, in addition to the frontal cortex region, similar to that observed in AD. In individuals exposed to air pollutants, there is a link between oxidative stress, neuroinflammation, and neurodegeneration, with the presence of hyper-phosphorylated tau and Aβ plaques in the frontal cortex. The air pollution can cause an increase in Aβ 42 formation, accumulation, and impaired cognitive function [ 77 , 78 ].
In recent years, the number of studies on the role of nutrition in AD have been increased. Several dietary supplements such as antioxidants, vitamins, polyphenols, and fish were reported to decrease the risk of AD, whereas saturated fatty acids and high-calorie intake were associated with increasing the risk of AD [ 79 ]. The food processing causes degradation of heat-sensitive micronutrients (e.g., vitamin C and folates), loss of large amounts of water, and formation of toxic secondary products (advanced glycation end products, AGEs) from non-enzymatic glycation of free amino groups in proteins, lipids, and nucleic acids. The toxic effect of AGEs is referred to as their ability to induce oxidative stress and inflammation by modifying the structure and function of the cell surface receptors and body proteins. Different studies demonstrated that elevated AGEs serum level is associated with cognitive decline and progression of AD. The AGE receptor (RAGE) is located in different places within the body, including microglia and astrocytes, and was established to be overexpressed in the brain of AD patients and serve as a transporter and a cell surface receptor for Aβ [ 80 ]. Malnutrition is another risk factor for AD. Deficiency in nutrients such as folate, vitamin B12, and vitamin D may cause a decrease in cognitive function, in addition to the fact that patients with AD suffer from problems associated with eating and swallowing, which may increase the risk of malnutrition [ 81 ].
Metals are found in nature and biological systems and can be divided into bio-metals that have a physiological function in living organisms (e.g., copper, zinc, and iron), and toxicological metals which do not possess any biological function (e.g., aluminum and lead) [ 82 ]. Aluminum is used significantly in the industries such as processed foods, cosmetics, medical preparations, medicines, and others. In the body, aluminum is bound to plasma transferrin and to citrate molecules that can mediate the transfer of aluminum to the brain. Studies demonstrated that Al accumulates in the cortex, hippocampus, and cerebellum areas, where it interacts with proteins and causes misfolding, aggregation, and phosphorylation of highly phosphorylated proteins like tau protein, characteristic of AD [ 83 ]. Lead competes with the binding site of bio-metals like calcium and can cross the blood–brain barrier (BBB) rapidly, where it can modify neural differentiation and synaptogenesis and cause severe damage. Studies revealed that an acute exposure to lead was associated with AD and caused an increase of β-secretase expression and Aβ accumulation. Cadmium is a carcinogenic water-soluble metal that can cross the BBB and cause neurological diseases like AD. Results have demonstrated that Cadmium ions are involved in the aggregation of Aβ plaques and the self-aggregation of tau in the AD brain. The data accumulated on metals support the notion that they are among the risk factors involved in the development of AD [ 84 ].
Chronic infections to the central nervous system (CNS) can cause an accumulation of Aβ plaques and NFT, therefore, they are included among the risk factors in AD. Studies by Dr. Itzhaki showed that the DNA of herpes simplex virus (HSV-1) was found in patients with ApoE-ε4 allele carriers, which explains the high risk for developing AD. HSV-1 can replicate in the brain, which can result in the activation of the inflammatory response and an increase in Aβ deposition, resulting in damage to neurons and gradual development of AD. On the other hand, the study results by Miklossy and Balin’s have revealed the role of chronic bacterial infections in AD. For example, syphilitic dementia caused by spirochete bacteria ( Treponema pallidum ), which are accumulated in the cerebral cortex, produced lesions similar to neurofibrillary tangles, which led to devastating neurodegenerative disorders. Besides, Chlamydia pneumonia bacterium can trigger late-onset AD by activation of astrocyte and cytotoxic microglia, disrupt calcium regulation and apoptosis, resulting in deterioration of cognitive function, and increase the risk of AD [ 85 , 86 , 87 ].
5.2.4. Medical Factors
Several risk factors are related to the development of Alzheimer’s disease. Adding to this list, older people with AD usually have medical conditions such as cardiovascular disease (CVD), obesity, diabetes, and others. All of these conditions are associated with increased risk of AD [ 88 , 89 ].
Cardiovascular Disease (CVDs)
CVDs are recognized as an important risk factor for AD, such as the stroke that is associated with increased risk of dementia due to a neural tissue loss, which enhances degenerative effect and influences amyloid and tau pathology. Atrial fibrillation also causes embolisms which leads to stroke and a decrease in memory and cognitive functions. Moreover, heart failure affects the pumping function of the heart and results in insufficient blood supply to the body and hypo-perfusion of the brain that leads to hypoxia and neural damage. The coronary heart disease’s hypothesis indicates that atherosclerosis, peripheral artery disease, hypo-perfusion, and emboli are all related to increased risk of AD. Hypertension is associated with thickening of vessel walls and narrowing of the lumen which reduce the cerebral blood flow, and in chronic cases, it may cause cerebral edema, which all participate as risk factors for AD and CVD. The CVD is a modifiable risk factor and by focusing on its relationship with AD, a pathway to prevent and delay the disease can be obtained [ 89 , 90 ].
Obesity and Diabetes
Obesity is a term used for too much body fat in individuals due to consuming more calories than they burn and can be calculated by using the body mass index (BMI). Increasing the body fat is associated with a decreased brain blood supply which promotes brain ischemia, memory loss, and vascular dementia. The obesity, unhealthy diet, and other factors can cause impaired glucose tolerance (IGT) or diabetes, which is characterized by hyperglycemia that affects peripheral tissues and blood vessels. Chronic hyperglycemia can induce cognitive impairment as a result of increasing amyloid-beta accumulation, oxidative stress, mitochondrial dysfunction, and neuroinflammation. Obesity is characterized by increasing pro-inflammatory cytokines secretions from adipose tissue, which stimulate macrophages and lymphocytes and eventually lead to local and systemic inflammation. This inflammation promotes insulin resistance, hyperinsulinemia, and as a consequence, hyperglycemia. Obesity is a well-known risk factor for type 2 diabetes, CVDs, and cancer, which are identified as risk factors for dementia and AD. The brain inflammation causes an increase in microglia and results in reduced synaptic plasticity and impaired neurogenesis. Microglia can affect insulin receptor substrate 1 (IRS-1) and block intracellular insulin signaling, which has an important role in neural health. Therefore, alteration in insulin action can result in Aβ accumulation and reduce the tau protein degradation associated with AD [ 91 , 92 , 93 , 94 ].
6. Treatment
Currently, Alzheimer’s disease cases worldwide are reported to be around 24 million, and in 2050, the total number of people with dementia is estimated to increase 4 times. Even though AD is a public health issue, as of now, there is only two classes of drugs approved to treat AD, including inhibitors to cholinesterase enzyme (naturally derived, synthetic and hybrid analogues) and antagonists to N -methyl d -aspartate (NMDA). Several physiological processes in AD destroy Ach-producing cells which reduce cholinergic transmission through the brain. Acetylcholinesterase inhibitors (AChEIs), which are classified as reversible, irreversible, and pseudo-reversible, act by blocking cholinesterase enzymes (AChE and butyrylcholinesterase (BChE)) from breaking down ACh, which results in increasing ACh levels in the synaptic cleft [ 95 , 96 , 97 ]. On the other hand, overactivation of NMDAR leads to increasing levels of influxed Ca 2+ , which promotes cell death and synaptic dysfunction. NMDAR antagonist prevents overactivation of NMDAR glutamate receptor and hence, Ca 2+ influx, and restores its normal activity. Despite the therapeutic effect of these two classes, they are effective only in treating the symptoms of AD, but do not cure or prevent the disease [ 98 , 99 ]. Unfortunately, only a few clinical trials on AD have been launched in the last decade and their outcome was a big failure. Several mechanisms have been proposed to understand AD pathology in order to modify its pathway and develop successful treatments, which include abnormal tau protein metabolism, β-amyloid, inflammatory response, and cholinergic and free radical damage [ 30 , 100 ]. On the other hand, most AD modifiable risk factors such as cardiovascular or lifestyle habits can be prevented without medical intervention. Studies showed that physical activity can improve the brain health and reduce AD by activating the brain vascularization, plasticity, neurogenesis, and reducing inflammation by decreasing Aβ production, which all result in improving cognitive function in older people. Moreover, the Mediterranean diet (MD), intellectual activity, and higher education all may reduce the progression of AD and memory loss and increase the brain capacity and cognitive functions. Several studies revealed that multi-domain intervention which includes lifestyle (diet, exercise, and cognitive training), depression of AD symptoms, and controlling cardiovascular risk factors, can increase or maintain cognitive function and prevent new cases of AD in older people [ 101 ]. Herein, we summarize the currently available drugs and theories for the development of new therapies for AD.
6.1. Symptomatic Treatment of AD
6.1.1. cholinesterase inhibitors.
According to the cholinergic hypothesis, AD is due to the reduction in acetylcholine (ACh) biosynthesis. Increasing cholinergic levels by inhibiting acetylcholinesterase (AChE) is considered one of the therapeutic strategies that increases cognitive and neural cell function. AChEIs are used to inhibit acetylcholine degradation in the synapses, which results in continuous accumulation of ACh and activation of cholinergic receptors. Tacrine (tetrahydroaminoacridine) ( 1, Figure 4 ) was the first FDA (Food and Drug Administration)-approved cholinesterase inhibitor drug for the treatment of AD, which acts by increasing ACh in muscarinic neurons, but it exited the market immediately after its introduction due to a high incidence of side effects like hepatotoxicity and a lack of benefits, which was observed in several trials. Later on, several AChEIs were introduced, such as donepezil ( 2 , Figure 4 ), rivastigmine ( 3 , Figure 4 ), and galantamine ( 4 , Figure 4 ), and are currently in use for the symptomatic treatment of AD [ 34 , 97 , 102 , 103 ]. Another strategy that may help in the treatment of AD is increasing choline reuptake and as a result, increasing acetylcholine synthesis at the presynaptic terminals. This can be achieved by targeting choline transporter (CHT1) which is responsible for supplying choline for the synthesis of ACh. Developing drugs that are capable of increasing CHT1 at the plasma membrane may become the future therapy of AD [ 36 ].
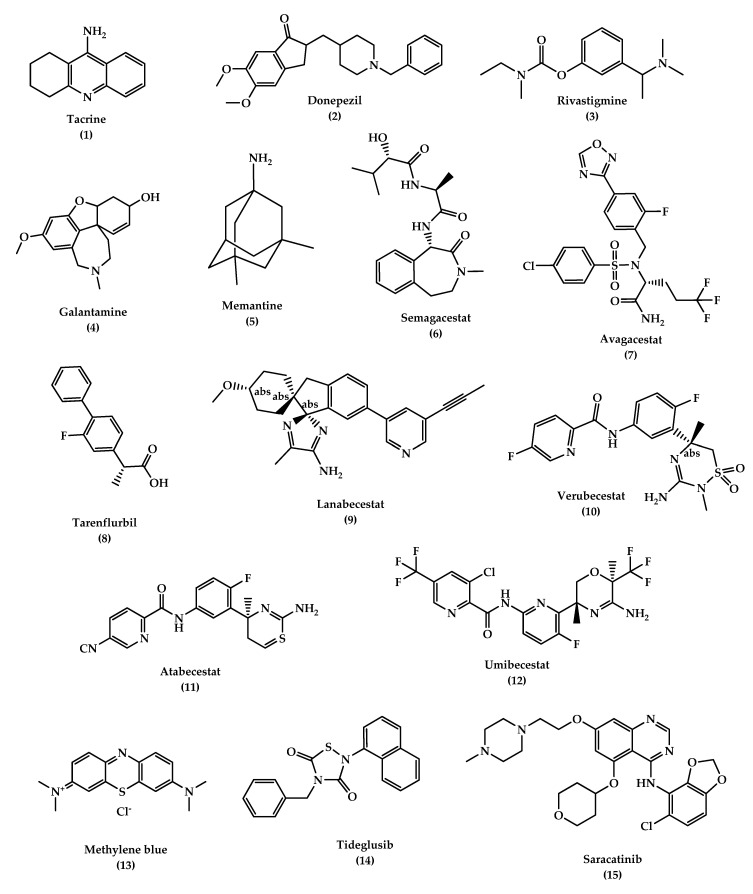
The chemical structures of approved drugs for symptomatic treatment of AD (tacrine 1 , donepezil 2 , rivastigmine 3 , galantamine 4 , and memantine 5 ) and disease-modifying compounds that entered clinical trials (semagacestat 6 , avagacestat 7 , tarenflurbil 8 , lanabecestat 9 , verubecestat 10 , atabecestat 11 , umibecestat 12 , methylene blue 13 , tideglusib 14 , and saracatinibin 15 ).
Donepezil ( 2 , Figure 4 ) is an indanonebenzylpiperidine derivative and a second generation of AChEIs and is considered the leading drug for AD treatment. Donepezil binds to acetylcholinesterase reversibly and inhibits acetylcholine hydrolysis, which leads to a higher concentration of ACh at the synapses. The drug is well-tolerated with mild and transient cholinergic side effects which are related to the gastrointestinal and nervous systems. It should be noted that donepezil is used to treat symptoms of AD such as improving cognition and behavior without altering the AD progression [ 104 , 105 , 106 ].
Rivastigmine
Rivastigmine ( 3 , Figure 4 ) is a pseudo irreversible inhibitor of AChE and butyrylcholinesterase (BuChE) that acts by binding to the two active sites of AChE (anionic and estearic sites), which results in preventing ACh metabolism. BuChE is found mostly in glial cells with only 10% of AChE activity in the normal brain, whereas in the AD brain, its activity is increased to 40–90%, while ACh activity is reduced simultaneously, which suggests that BuChE action may indicate a moderate to severe dementia. Rivastigmine dissociates more slowly than AChE, which is why it is called a pseudo-irreversible, and it undergoes metabolism at the synapse by AChE and BuChE. The drug is used in mild to moderate AD cases. It improves cognitive functions and daily life activities. Oral administration of the drug is associated with adverse effects such as nausea, vomiting, dyspepsia, asthenia, anorexia, and weight loss. In many cases, these side effects are the main reason behind stopping taking the medicine, however, they can be settled down in time and consequently, the drug becomes more tolerated. Rivastigmine can be delivered by transdermal patches for controlled and continuous delivery of the drug through the skin, with enhanced tolerability and caregiver satisfaction. Also, the patches can deliver a lower dosage compared to pills, which results in reduced side effects. Most AD patients suffer from memory loss and swallowing problems which affect their compliance in administering oral drugs at regular intervals. Therefore, the use of transdermal patches is the most appropriate method for delivering the drug in AD patients [ 107 , 108 , 109 , 110 ].
Galantamine (GAL)
Galantamine ( 4 , Figure 4 ) is considered a standard first-line drug for mild to moderate AD cases. GAL is a selective tertiary isoquinoline alkaloid with a dual mechanism of action in which it acts as a competitive inhibitor of AChE and can bind allosterically to the α-subunit of nicotinic acetylcholine receptors and activate them. GAL can improve behavioral symptoms, daily life activities, and cognitive performance with good efficacy and tolerability, similar to other AChE inhibitors. Several delivery systems were developed to improve the drug delivery to the brain: Wahba et al. attached GAL to ceria-containing hydroxyapatite particles for selective delivery of the drug to the affected regions in the brain. Misra et al. and Fornaguera et al. used solid-lipid nanoparticles and nano-emulsification approaches respectively, to carry GAL hydrobromide. The results of these studies demonstrated a promising strategy for safe delivery of the drug. Hanafy et al. developed nasal GAL hydrobromide/chitosan complex nanoparticles which showed good pharmacological efficacy, while Woo et al. utilized the patch system as a carrier for a controlled release dosage form of the drug [ 111 , 112 , 113 , 114 ].
6.1.2. N -methyl d -aspartate (NMDA) Antagonists
NMDAR is believed to have a dominant role in the pathophysiology of AD. NMDAR stimulation results in Ca 2+ influx which activates signal transduction and as a consequence, it triggers gene transcription essential for the formation of a long-term potentiation (LTP), which is important for synaptic neurotransmission, plasticity, and memory formation. Over-activation of NMDARs causes an abnormal level of Ca 2+ signaling and overstimulation of glutamate, which is the primary excitatory amino acid in the CNS, which results in excitotoxicity, synaptic dysfunction, neuronal cell death, and a decline in cognitive functions. Several NMDAR uncompetitive antagonists have been developed and entered clinical trials, however, most of them failed due to low efficacy and side effects. Memantine ( 5 , Figure 4 ) is the only approved drug in this category to treat moderate to severe AD; in addition, other NMDAR uncompetitive antagonist compounds are being developed, such as RL-208 (3,4,8,9-tetramethyltetracyclo [4.4.0.0 3,9 .0 4,8 ]dec-1-yl)methylamine hydrochloride), a polycyclic amine compound that may possess a promising therapeutic effect in age-related cognitive problems and AD [ 115 , 116 , 117 ].
Memantine ( 5 , Figure 4 ) is a low-affinity uncompetitive antagonist of the NMDAR, a subtype of glutamate receptor that prevents over-activation of the glutaminergic system involved in the neurotoxicity in AD cases. Memantine is used for the treatment of moderate to severe AD alone or in combination with AChEI. The drug is safe and well-tolerated, it blocks the excitatory receptor without interfering with the normal synaptic transmission due to memantine’s low affinity, where it is displaced rapidly from NMDAR by high concentrations of glutamate, thus avoiding a prolonged blockage. The latter is associated with high side effects, especially on learning and memory [ 99 , 118 ].
6.2. Promising Future Therapies
6.2.1. disease-modifying therapeutics (dmt).
Disease-modifying treatment or therapy (DMT) alter the progression of AD by working on several pathophysiological mechanisms. This is in contrast to symptomatic therapy which works on improving the cognitive functions and decreasing symptoms such as depression or delusions without affecting or modifying the disease. DMTs, either immunotherapies or small molecules, are administrated orally and are being developed to prevent AD or decrease its progression. Several DMTs have been developed and entered the clinical trials, such as AN-1792, a synthetic Aβ peptide (human Aβ 1–42 peptide of 42-amino acids with the immune adjuvant QS-21) and the first active immunotherapy for AD which entered phase II clinical trials and discontinued due to a meningoencephalitis side effect in 6% of the patients. Other drugs were also developed and failed in the clinical trials, including the anti-Aβ antibody (solanezumab and bapineuzumab), γ-Secretase inhibitors (semagacestat 6 , avagacestat 7 , and tarenflurbil 8 ) ( Figure 4 ) and β-secretase inhibitors (BACE) (Lanabecestat 9, verubecestat 10 , and atabecestat 11 ) ( Figure 4 ). DMTs failures are due to several factors, such as starting therapy too late, giving treatment for the wrong main target, use of inappropriate drug doses, and misunderstanding of the pathophysiology of AD. Several immunotherapies described in Table 1 have been developed over decades, including: CAD106, an active Aβ immunotherapy that induces Aβ antibodies in animal models and consists of multiple copies of Aβ1–6 peptide coupled to Qβ coat protein, a virus-like particle, and is still in clinical trials, and CNP520 (umibecestat, 12 ) ( Figure 4 ), a small molecule that inhibits beta-scretase-1 (BACE-1) and therefore inhibits Aβ production. CNP520 was found to reduce Aβ plaque deposition and Aβ levels in the brain and CSF in rats, dogs, and healthy adults ≥ 60 years old, and is still under clinical trials. Furthermore, aducanumab, gantenerumab, and crenezumab are all human Aβ monoclonal antibody that bind with high affinity to aggregated Aβ, and they are still under study in the clinical phases with other DMTs described in Table 1 [ 6 , 119 , 120 , 121 , 122 , 123 , 124 ].
Disease modifying agents for the treatment of Alzheimer’s disease in clinical trials.
Another class targeting the α-secretase enzyme was developed and has been considered as therapeutic agents. α-secretase modulators or activators stimulate the cleavage of APP. There is little knowledge about the activation pathway, but research assumes that it is promoted by the phosphatidylinositol 3-kinase (PI3K)/Akt pathway or by γ-aminobutyric acid (GABA) receptor signaling. Targeting these pathways may give potential therapeutic agents for AD [ 6 ].
In addition to the anti-amyloid agents, the tau aggregation inhibitors are another promising DMT. The tau is a biomarker for neurofibrillary tangles (NFT) in AD and naturally modulates microtubule stability, signaling pathways, and axonal transport. A modification in tau conformation results in toxic aggregation. Therefore, the prevention of tau aggregation becomes an interesting approach for drug discovery to reduce AD progression. Studies in mice have shown that tau oligomers cause mitochondrial damage, disruption of neuronal signaling, synaptic loss, and memory impairment. Disease-modifying therapeutics (DMT) like small molecules can be used to inhibit the initial step in the tau aggregation and thereby reduce its accumulation. Methylene blue ( 13 , Figure 4 ) is a blue dye that inhibits the tau aggregation and entered phase II clinical trials to treat mild to moderate AD. Upon administration of the drug, the color of the urine becomes blue, which indicates a lack of binding, and because of that, the study was highly criticized. Other approaches suggest that an inhibition of specific kinases such as glycogen synthase kinase 3 (GSK3β) can inhibit tau hyperphosphorylation and block tau deposition. Examples of these entities include tideglusib ( 14 , or NP-031112 (NP-12), Figure 4 ), a thiazolidinedione-derived compound, lithium, pyrazolopyridines, pyrazolopyrazines, sodium valproate, and others. Another protein kinase inhibitor is saracatinib (AZD0530) ( 15 , Figure 4 ), which acts by inhibiting tyrosine kinase and has shown good results in improving memory in transgenic mice and is currently in phase II trials [ 125 , 126 , 127 ]. Davidowitz et al. utilized the hatu mouse model of tauopathy to study the efficacy of a lead small molecule in preventing tau accumulation. The study results demonstrated a significant reduction in tau levels and its phosphorylated form levels, which indicates the ability to inhibit the entire pathway of the tau aggregation by using an optimized lead compound [ 128 ].
6.2.2. Chaperones
Protein misfolding caused by mutations or environmental factors results in aggregations that are toxic, and their accumulation causes neurodegenerative disorders like AD. Naturally, cells develop protein quality control (PQC) systems that inhibit protein misfolding before exerting their toxic effects. With age, this balance is altered and the misfolded shapes overwhelm the PQC system, which in turn activates the unfolded protein response (UPR) that stops the protein synthesis and increases chaperone production. Generally, the cells in humans have proteins that are responsible for other proteins to function and arrive to their destination in the cell. These proteins are called “chaperones”. Chaperones are involved in protein folding and improvement of the PQC system efficiency. Therefore, it is considered a promising candidate for treating neurodegenerative diseases. It can be classified into three groups: (1) molecular chaperones, which are proteins that assist other nonnative proteins in their folding or unfolding, like overexpression of heat shock proteins (Hsps) that serve as neuroprotective agents, (2) pharmacological chaperones, which are low molecular weight compounds (enzymes or receptor-ligand or selective binding molecules) that induce refolding of proteins, stabilize their structure, and restore their function, and (3) chemical chaperones, also low molecular weight compounds, which are divided into two groups, osmolytes and hydrophobic compounds. The members in these two groups have no specific mechanism of action and need high concentrations to exert their therapeutic effects [ 129 ].
Heat Shock Proteins (Hsps)
The causes for most neurodegenerative diseases are protein misfolding and aggregation, which lead to cell death. The molecular chaperone can be intracellular, such as in the case of heat shock proteins (e.g., Hsp40, Hsp60, Hsp70, Hsp90, Hsp100, and Hsp110), and extracellular, such as clustering and alpha-macroglobulin. HSPs play an essential role in the protein folding process and protect cells from harmful stress-related events. There are two families of Hsps: (a) classic Hsps that possess an ATP-binding site with a molecular weight of 60 kD or more. This family includes Hsp100, Hsp90, Hsp70, and Hsp60, and (b) the small Hsps such as αB-crystalline, Hsp27, Hsp20, HspB8, and HspB2/B3 that lack ATP-binding site, with a molecular weight of 40 kD or less. These proteins can assist other Hsps in their refolding function. Failure of these mechanisms can lead to oxidative stress, mitochondrial dysfunction, and many other conditions that cause damage, a loss of neurons, and a progression of neurodegenerative diseases. Different HSPs can block the aggregation process of misfolded proteins, like amyloidogenic proteins (Aβ and tau), and promote their degradation [ 130 , 131 ].
Hsp60 plays an important role in mitochondrial protein folding. Its role in AD is not clear, some believe that the protein has a protective role and others think it has a harmful effect where it can be over-expressed by activated microglia, which increases pro-inflammatory factors such as toll-like receptor 4 (TLR-4) that stimulate neuronal cell death. Therefore, inhibiting activated microglia and Hsp60 expression is a promising strategy for preventing neurodegenerative diseases. Examples of compounds that inhibit Hsp60 are mizoribine (Immunosuppressant) ( 16 , Figure 5 ) and pyrazolopyrimidine EC3016 ( 17 , Figure 5 ). Both compounds act by blocking ATPase activity of Hsp60 and inhibiting protein folding. On the other hand, avrainvillamide, a fungal metabolite ( 18 , Figure 5 ), and epolactaene, a bacterial metabolite ( 19 , Figure 5 ), act by binding to the Hsp60′s cysteine residues and inhibit its folding activity. However, Hsp60’s role in AD remains controversial and there is a need for more investigations to understand its role [ 130 ].
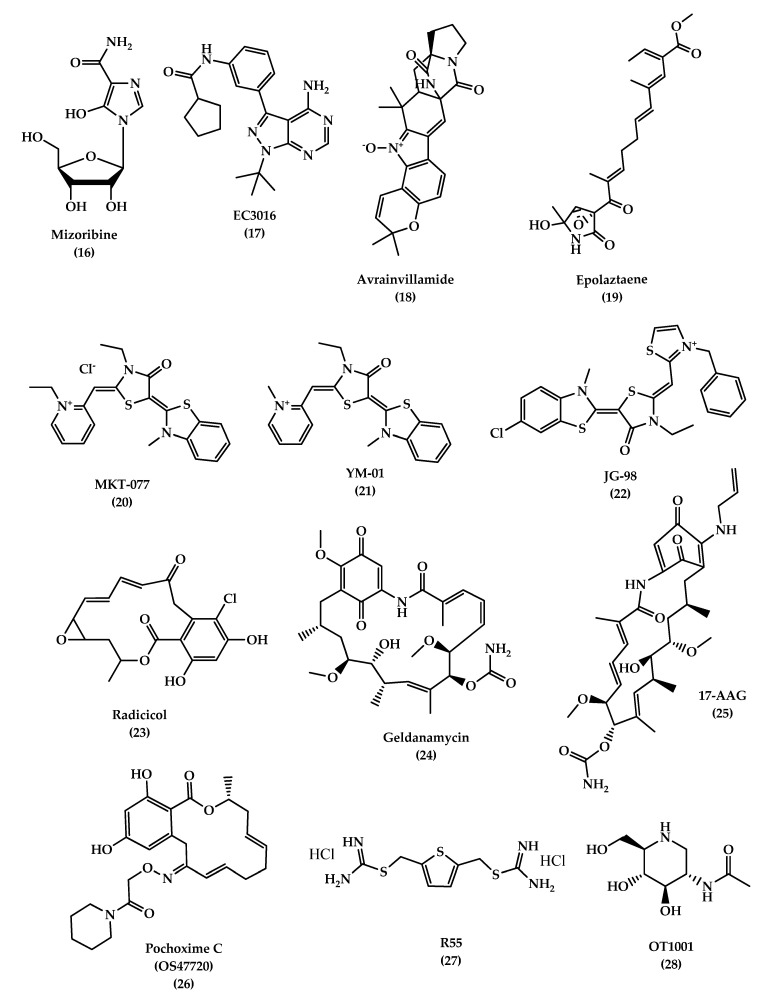
The chemical structures of different chaperone molecules: Mizoribine 16 , EC3016 17 , Avrainvillamide 18 , Epolaztaene 19 , MKT-077 20 , YM-01 21 , JG-98 22 , Radicicol 23 , Geldanamycin 24 , 17-AAG 25 , Pochoxime C (OS47720) 26 , R55 27 , and OT1001 28 .
Studies have shown that Hsp70 binds to Aβ42 and prevents self-aggregation. Martín-Peña et al. studied two isoforms of Hsp70, cytosolic and extracellular, in Drosophila flies AD models and evaluated their protective role against memory decline that results from Aβ42 aggregation. The animal studies showed that Hsp70 has a dual function: intracellularly and extracellularly, where it protects against Aβ42 neurotoxicity and synaptic loss. In addition to its ability to bind to tau and its hyper-phosphorylated form and prevent its formation, it decreases aggregation and promotes tau binding to microtubules. Hsp70 acts by activating microglia, insulin-degrading enzyme, and tumor growth facto r- β1, which degrades β-amyloids and prevents memory impairments [ 132 , 133 ]. Some studies in AD brain tissue demonstrated an overexpression of Hsp70 levels and a correlation with the presence of activated glia and stressed neurons. Also, it was found that Hsp70 is associated with extracellular deposits in AD. Drug therapies targeting Hsp70, mainly referring to previous anticancer drugs which target and inhibit Hsp70 ATP-binding site, are considered as candidates in AD treatment due to their ability to reduce tau levels in vitro and ex vivo. MKT-077(1-ethyl-2-(( Z )-(( E )-3-ethyl-5-(3-methylbenzo [ d ]thiazol-2(3 H )-ylidene)-4-oxothiazolidin-2-ylidene)methyl)pyridin-1-ium chloride) ( 20 , Figure 5 ), is an anticancer rhodacyanine compound that binds to mortalin, a mitochondrial Hsp70 site, and acts as an anti-proliferative agent, but the use of this compound was stopped due to toxicity side effects and low BBB penetration. On the other hand, YM-01 ( 21 , Figure 5 ), a more potent MKT-077 derivative, was developed with a single replacement of the ethyl group on the pyridinium nitrogen of MKT-077 with a methyl group. JG-98 ( 22 , Figure 5 ) is also an MKT-077 derivative with a 60-fold higher binding affinity to Hsp70 than YM-01 [ 130 , 134 , 135 , 136 ].
Hsp90 is another type of HSP that regulates the tau phosphorylation and dephosphorylation. An inhibition of Hsp90 results in a decrease in phosphorylation of tau due to a reduction in tau kinases, which is thought to be responsible for tau pathogenesis when it is hyperactivated. Hsp90 inhibitors are used for cancer therapy, but recently, they are considered as promising therapy for AD. Radicicol (RDC) ( 23 , Figure 5 ) and geldanamycin (GA) ( 24 , Figure 5 ) are Hsp90 inhibitors. GA is a natural antifungal compound and the first discovered Hsp90 inhibitor. Studies on this inhibitor were stopped due to its toxicity. On the other hand, 17-AAG (17-(Allylamino)-17-demethoxygeldanamycin) ( 25 , Figure 5 ) is a GA derivative with a lower toxicity and better pharmacokinetic profile that showed a good improvement of the cognitive function by inducing other HSPs, like Hsp70, in addition to reducing NFTs in the transgenic mouse model by blocking the tau phosphorylation pathway, indirectly [ 137 , 138 ]. Pochoxime C (OS47720) ( 26 , Figure 5 ) is also a CNS-permeable Hsp90 inhibitor that showed good safety and efficacy profiles when tested in the AD mouse model. Studies revealed that OS47720 acts by strengthening synaptic function via heat shock factor (HSF-1) activation and dependent transcriptional events [ 139 ].
The combined studies demonstrate that targeting HSPs is a promising strategy to develop drugs with a new mechanism of action for reducing pathogenic tau levels and restoring normal tau homeostasis.
Vacuolar sorting protein 35 (VPS35)
An accumulation of proteins in neurons and glial cells leads to disturbance of cellular protein homeostasis. The endosomal-lysosomal system is responsible for transporting proteins for recycling and degradation. Any malfunction in the system can lead to several diseases, such as Alzheimer’s disease. Retromer is a complex of regulator proteins composed of sorting nexin (SNX1, 2, 5, 6) and vacuolar sorting proteins (VPS 26, 29, 35), which are responsible for transporting cargo molecules from the endosome to the trans -Golgi network. A loss of retromer’s function results in the downregulation of VPS35, which can increase Aβ formation, induce cognitive impairments, and cause synaptic dysfunction, which is reported in AD patients [ 140 , 141 ]. A study on 3xTg mice brains was conducted to evaluate the effect of VPS35 overexpression on memory function. The study showed that a significant reduction of the Aβ peptide and tau neuropathology (soluble, insoluble, and phosphorylated tau) was associated with overexpression of VPS35, in addition to a reduction in neuroinflammation and ameliorating synaptic dysfunction [ 142 ]. Therefore, VPS35 is an important promising therapeutic target for AD treatment. A small pharmacological chaperones molecule called R55 (thiophene-2,5-diylbis(methylene) dicarbamimidothioatedihydrochloride) ( 27 , Figure 5 ), a thiophenethiourea derivative, can enhance retromer stability and function by increasing retromer proteins, shifting AOO from the endosome, and reducing pathogenic processing of APP, which may serve as a promising therapeutic molecule for neurodegenerative diseases [ 143 ].
Studies demonstrated that the accumulation of gangliosides has been associated with misfolding and aggregation of proteins in neurodegenerative diseases. Abnormal levels of mono-sialoganglioside (GM1, GM2, and GM3) have been reported in AD brains. Mutant forms of Aβ, like Dutch mutant APPE693Q, showed susceptibility to pro-aggregation properties of GM2 and GM3, resulting in the formation of Aβ peptides complexes with gangliosides (ganglioside-bound Aβ (GAβ) peptide) and subsequently leading to an acceleration of aggregation and accumulation of Aβ peptides.
β-hexosaminidase (β-hex) is a lysosomal enzyme that acts by catabolizing GM2 ganglioside, and increasing its activity can lead to a reduction of GM2 levels and Aβ aggregation and accumulation. Small molecules like pharmacological chaperones (PC) can selectively bind and stabilize wild-type proteins and restore their normal folding. OT1001 ( 28 , Figure 5 ) is an iminosugar PC that targets β-hex and increases its level in the brain and reduces GAβ pathology. Studies on Dutch APPE693Q transgenic mice showed that OT1001 has good pharmacokinetics, brain penetration ability, and tolerability, with lower side effects. These make the compound a good drug candidate for increasing the β-hex activity [ 144 ].
6.2.3. Natural Extract
For a long time, natural compounds have been used as therapeutic agents for several pathological diseases, and recent studies showed that they possess a neuroprotective effect. In vitro and in vivo studies have proven that natural compounds possess a therapeutic potential for AD, which allowed some of them to enter the clinical trials stages. Nicotine was the first natural compound entered in the clinical trials for AD, then other compounds like vitamins C, E, and D gained more attention and interest due to their protective role against neuroinflammation and oxidative damage. Recently, bryostatin, a macrolide lactone extract from bryozoan Bugula neritina, has been evaluated and showed the ability to induce α-secretase activity, reduce Aβ production, and enhance the learning and memory in an AD mice model [ 145 ]. Other natural compounds used in folk medicine (traditional Chinese medicine (TCM)) demonstrated a great potential in treating AD by acting on several mechanisms, as shown in Table 2 below [ 146 ].
Natural compounds used in folk medicine and their mechanism of actions.
7. Conclusions
Alzheimer’s disease is now considered a world health concern; as a consequence, the National Institute on Aging—Alzheimer’s Association reclassified and updated the 1984 NINCDS-ADRDA criteria for higher specificity, sensitivity, and early identification of patients at risk of developing AD. Several criteria have been proposed for a more accurate diagnosis of AD, including clinical biomarkers, bodily fluids, and imaging studies. Despite that, the treatment of AD remains symptomatic, without alteration in the disease’s prognosis. Inhibitors to cholinesterase enzyme such as galantamine, donepezil, and rivastigmine, and NMDA antagonists such as memantine, improve memory and alertness but do not prevent progression. Several studies have shown that modification in lifestyle habits like diet and exercise can improve brain health and reduce AD without medical intervention and is considered as a first-line intervention for all AD patients. Recently, the research is focusing on targeting the pathological features of AD such as Aβ and p-tau. Future therapies such as disease-modifying treatment can alter the progression of AD by targeting the Aβ pathway, and many drugs have entered the clinical trials, like AN-1792, solanezumab, bapineuzumab, semagacestat, avagacestat, and tarenflurbil, but failed in demonstrating efficacy in the final clinical stages. Other DMTs are still under investigation, such as those targeting Aβ and tau pathologies, such as aducanumab, gantenerumab, crenezumab, tideglusib, lithium, and others. Other promising compounds called chaperones like heat shock proteins and vacuolar sorting protein 35 (VPS35) function by assisting other proteins to function normally and to arrive at their destination in the cell safely, and therefore can be used as a treatment for neurodegenerative diseases. Moreover, the natural extracts used in folk Chinese medicine showed great potential in treating AD by acting on several mechanisms’ pathways. In conclusion, the success of AD treatment depends on its early administration and patient monitoring for disease progression using biomarkers diagnosis. Future therapies that target tau pathology and the use of combination therapy may have a potential to slow the progression of AD pathology. Designing a potent, selective, and effective drug is urgently needed to treat patients with AD and those at risk for developing the disease.
Author Contributions
Literature survey and first draft writing were done by Z.B., and final draft, including the revisions, were accomplished by R.K. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. De-Paula V.J., Radanovic M., Diniz B.S., Forlenza O.V. Alzheimer’s disease. Sub-Cell. Biochem. 2012;65:329–352. doi: 10.1007/978-94-007-5416-4_14. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Cipriani G., Dolciotti C., Picchi L., Bonuccelli U. Alzheimer and his disease: A brief history. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2011;32:275–279. doi: 10.1007/s10072-010-0454-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Blass J.P. Alzheimer’s disease. Dis. A Mon. Dm. 1985;31:1–69. doi: 10.1016/0011-5029(85)90025-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Terry R.D., Davies P. Dementia of the Alzheimer type. Annu. Rev. Neurosci. 1980;3:77–95. doi: 10.1146/annurev.ne.03.030180.000453. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Rathmann K.L., Conner C.S. Alzheimer’s disease: Clinical features, pathogenesis, and treatment. Drug Intell. Clin. Pharm. 1984;18:684–691. doi: 10.1177/106002808401800902. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Yiannopoulou K.G., Papageorgiou S.G. Current and future treatments in alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020:12. doi: 10.1177/1179573520907397. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Schachter A.S., Davis K.L. Alzheimer’s disease. Dialogues Clin. Neurosci. 2000;2:91–100. doi: 10.1007/s11940-000-0023-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Jatoi S., Hafeez A., Riaz S.U., Ali A., Ghauri M.I., Zehra M. Low Vitamin B12 levels: An underestimated cause of minimal cognitive impairment and dementia. Cureus. 2020;12:e6976. doi: 10.7759/cureus.6976. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Cho H.S., Huang L.K., Lee Y.T., Chan L., Hong C.T. Suboptimal baseline serum Vitamin B12 is associated with cognitive decline in people with Alzheimer’s disease undergoing cholinesterase inhibitor treatment. Front. Neurol. 2018;9:325. doi: 10.3389/fneur.2018.00325. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Neugroschl J., Wang S. Alzheimer’s disease: Diagnosis and treatment across the spectrum of disease severity. Mt. Sinai J. Med. N. Y. 2011;78:596–612. doi: 10.1002/msj.20279. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Mayeux R., Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006239. doi: 10.1101/cshperspect.a006239. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Yaari R., Fleisher A.S., Tariot P.N. Updates to diagnostic guidelines for Alzheimer’s disease. Prim. Care Companion Cns Disord. 2011;13:11f01262. doi: 10.4088/PCC.11f01262. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Spires-Jones T.L., Hyman B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82:756–771. doi: 10.1016/j.neuron.2014.05.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Singh S.K., Srivastav S., Yadav A.K., Srikrishna S., Perry G. Overview of Alzheimer’s disease and some therapeutic approaches targeting abeta by using several synthetic and herbal compounds. Oxidative Med. Cell. Longev. 2016;2016:7361613. doi: 10.1155/2016/7361613. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Cras P., Kawai M., Lowery D., Gonzalez-DeWhitt P., Greenberg B., Perry G. Senile plaque neurites in Alzheimer disease accumulate amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 1991;88:7552–7556. doi: 10.1073/pnas.88.17.7552. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Perl D.P. Neuropathology of Alzheimer’s disease. Mt. Sinai J. Med. N. Y. 2010;77:32–42. doi: 10.1002/msj.20157. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Armstrong R.A. The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer’s disease. Folia Neuropathol. 2009;47:289–299. [ PubMed ] [ Google Scholar ]
- 22. Chen G.F., Xu T.H., Yan Y., Zhou Y.R., Jiang Y., Melcher K., Xu H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017;38:1205–1235. doi: 10.1038/aps.2017.28. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Tabaton M., Piccini A. Role of water-soluble amyloid-beta in the pathogenesis of Alzheimer’s disease. Int. J. Exp. Pathol. 2005;86:139–145. doi: 10.1111/j.0959-9673.2005.00428.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Brion J.P. Neurofibrillary tangles and Alzheimer’s disease. Eur. Neurol. 1998;40:130–140. doi: 10.1159/000007969. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Metaxas A., Kempf S.J. Neurofibrillary tangles in Alzheimer’s disease: Elucidation of the molecular mechanism by immunohistochemistry and tau protein phospho-proteomics. Neural Regen. Res. 2016;11:1579–1581. doi: 10.4103/1673-5374.193234. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Overk C.R., Masliah E. Pathogenesis of synaptic degeneration in Alzheimer’s disease and Lewy body disease. Biochem Pharm. 2014;88:508–516. doi: 10.1016/j.bcp.2014.01.015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Lleo A., Nunez-Llaves R., Alcolea D., Chiva C., Balateu-Panos D., Colom-Cadena M., Gomez-Giro G., Munoz L., Querol-Vilaseca M., Pegueroles J., et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid. Mol. Cell. Proteom. Mcp. 2019;18:546–560. doi: 10.1074/mcp.RA118.001290. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Tarawneh R., D’Angelo G., Crimmins D., Herries E., Griest T., Fagan A.M., Zipfel G.J., Ladenson J.H., Morris J.C., Holtzman D.M. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer Disease. JAMA Neurol. 2016;73:561–571. doi: 10.1001/jamaneurol.2016.0086. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S., Bakardjian H., Benali H., Bertram L., Blennow K., et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Kumar A., Sidhu J., Goyal A. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. [(accessed on 8 December 2020)]. Alzheimer Disease. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499922/ [ Google Scholar ]
- 31. Wattmo C., Minthon L., Wallin A.K. Mild versus moderate stages of Alzheimer’s disease: Three-year outcomes in a routine clinical setting of cholinesterase inhibitor therapy. Alzheimer’s Res. Ther. 2016;8:7. doi: 10.1186/s13195-016-0174-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Apostolova L.G. Alzheimer disease. Continuum. 2016;22:419–434. doi: 10.1212/CON.0000000000000307. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Armstrong R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019;57:87–105. doi: 10.5114/fn.2019.85929. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Anand P., Singh B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res. 2013;36:375–399. doi: 10.1007/s12272-013-0036-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Babic T. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;67:558. doi: 10.1136/jnnp.67.4.558. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Monczor M. Diagnosis and treatment of Alzheimer’s disease. Curr. Med. Chem. Cent. Nerv. Syst. Agents. 2005;5:5–13. doi: 10.2174/1568015053202723. [ DOI ] [ Google Scholar ]
- 38. Hampel H., Mesulam M.M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain A J. Neurol. 2018;141:1917–1933. doi: 10.1093/brain/awy132. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Paroni G., Bisceglia P., Seripa D. Understanding the amyloid hypothesis in Alzheimer’s disease. J. Alzheimer’s Dis. Jad. 2019;68:493–510. doi: 10.3233/JAD-180802. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Kametani F., Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018;12:25. doi: 10.3389/fnins.2018.00025. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Ricciarelli R., Fedele E. The amyloid cascade hypothesis in Alzheimer’s disease: It’s time to change our mind. Curr. Neuropharmacol. 2017;15:926–935. doi: 10.2174/1570159X15666170116143743. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Guerreiro R., Bras J. The age factor in Alzheimer’s disease. Genome Med. 2015;7:106. doi: 10.1186/s13073-015-0232-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Riedel B.C., Thompson P.M., Brinton R.D. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2016;160:134–147. doi: 10.1016/j.jsbmb.2016.03.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Bekris L.M., Yu C.E., Bird T.D., Tsuang D.W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010;23:213–227. doi: 10.1177/0891988710383571. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. Off. J. Am. Coll. Med Genet. 2016;18:421–430. doi: 10.1038/gim.2015.117. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Khanahmadi M., Farhud D.D., Malmir M. Genetic of Alzheimer’s disease: A narrative review article. Iran. J. Public Health. 2015;44:892–901. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Li N.M., Liu K.F., Qiu Y.J., Zhang H.H., Nakanishi H., Qing H. Mutations of beta-amyloid precursor protein alter the consequence of Alzheimer’s disease pathogenesis. Neural Regen. Res. 2019;14:658–665. doi: 10.4103/1673-5374.247469. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Tcw J., Goate A.M. Genetics of beta-Amyloid precursor protein in Alzheimer’s disease. Cold Spring Harb. Perspect. Med. 2017;7:a024539. doi: 10.1101/cshperspect.a024539. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Bi C., Bi S., Li B. Processing of mutant beta-amyloid precursor protein and the clinicopathological features of familial Alzheimer’s disease. Aging Dis. 2019;10:383–403. doi: 10.14336/AD.2018.0425. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Dai M.H., Zheng H., Zeng L.D., Zhang Y. The genes associated with early-onset Alzheimer’s disease. Oncotarget. 2018;9:15132–15143. doi: 10.18632/oncotarget.23738. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Zhao J., Liu X., Xia W., Zhang Y., Wang C. Targeting amyloidogenic processing of APP in Alzheimer’s disease. Front. Mol. Neurosci. 2020;13:137. doi: 10.3389/fnmol.2020.00137. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Cai Y., An S.S., Kim S. Mutations in presenilin 2 and its implications in Alzheimer’s disease and other dementia-associated disorders. Clin. Interv. Aging. 2015;10:1163–1172. doi: 10.2147/CIA.S85808. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Lanoiselee H.M., Nicolas G., Wallon D., Rovelet-Lecrux A., Lacour M., Rousseau S., Richard A.C., Pasquier F., Rollin-Sillaire A., Martinaud O., et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017;14:e1002270. doi: 10.1371/journal.pmed.1002270. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. Embo Rep. 2007;8:141–146. doi: 10.1038/sj.embor.7400897. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Kelleher R.J., 3rd, Shen J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2017;114:629–631. doi: 10.1073/pnas.1619574114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Walker E.S., Martinez M., Brunkan A.L., Goate A. Presenilin 2 familial Alzheimer’s disease mutations result in partial loss of function and dramatic changes in Abeta 42/40 ratios. J. Neurochem. 2005;92:294–301. doi: 10.1111/j.1471-4159.2004.02858.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Kim J., Basak J.M., Holtzman D.M. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Giau V.V., Bagyinszky E., An S.S., Kim S.Y. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2015;11:1723–1737. doi: 10.2147/NDT.S84266. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Koldamova R., Fitz N.F., Lefterov I. ATP-binding cassette transporter A1: From metabolism to neurodegeneration. Neurobiol. Dis. 2014;72 Pt A:13–21. doi: 10.1016/j.nbd.2014.05.007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Nordestgaard L.T., Tybjaerg-Hansen A., Nordestgaard B.G., Frikke-Schmidt R. Loss-of-function mutation in ABCA1 and risk of Alzheimer’s disease and cerebrovascular disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015;11:1430–1438. doi: 10.1016/j.jalz.2015.04.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Foster E.M., Dangla-Valls A., Lovestone S., Ribe E.M., Buckley N.J. Clusterin in Alzheimer’s disease: Mechanisms, genetics, and lessons from other pathologies. Front. Neurosci. 2019;13:164. doi: 10.3389/fnins.2019.00164. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Holler C.J., Davis P.R., Beckett T.L., Platt T.L., Webb R.L., Head E., Murphy M.P. Bridging integrator 1 (BIN1) protein expression increases in the Alzheimer’s disease brain and correlates with neurofibrillary tangle pathology. J. Alzheimer’s Dis. Jad. 2014;42:1221–1227. doi: 10.3233/JAD-132450. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Andrew R.J., De Rossi P., Nguyen P., Kowalski H.R., Recupero A.J., Guerbette T., Krause S.V., Rice R.C., Laury-Kleintop L., Wagner S.L., et al. Reduction of the expression of the late-onset Alzheimer’s disease (AD) risk-factor BIN1 does not affect amyloid pathology in an AD mouse model. J. Biol. Chem. 2019;294:4477–4487. doi: 10.1074/jbc.RA118.006379. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Soler-Lopez M., Badiola N., Zanzoni A., Aloy P. Towards Alzheimer’s root cause: ECSIT as an integrating hub between oxidative stress, inflammation and mitochondrial dysfunction. Hypothetical role of the adapter protein ECSIT in familial and sporadic Alzheimer’s disease pathogenesis. Bioessays News Rev. Mol. Cell. Dev. Biol. 2012;34:532–541. doi: 10.1002/bies.201100193. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Mi Wi S., Park J., Shim J.H., Chun E., Lee K.Y. Ubiquitination of ECSIT is crucial for the activation of p65/p50 NF-kappaBs in Toll-like receptor 4 signaling. Mol. Biol. Cell. 2015;26:151–160. doi: 10.1091/mbc.e14-08-1277. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Soler-Lopez M., Zanzoni A., Lluis R., Stelzl U., Aloy P. Interactome mapping suggests new mechanistic details underlying Alzheimer’s disease. Genome Res. 2011;21:364–376. doi: 10.1101/gr.114280.110. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Zhao L., Woody S.K., Chhibber A. Estrogen receptor beta in Alzheimer’s disease: From mechanisms to therapeutics. Ageing Res. Rev. 2015;24:178–190. doi: 10.1016/j.arr.2015.08.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Sundermann E.E., Maki P.M., Bishop J.R. A review of estrogen receptor alpha gene (ESR1) polymorphisms, mood, and cognition. Menopause. 2010;17:874–886. doi: 10.1097/gme.0b013e3181df4a19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Yaffe K., Lindquist K., Sen S., Cauley J., Ferrell R., Penninx B., Harris T., Li R., Cummings S.R. Estrogen receptor genotype and risk of cognitive impairment in elders: Findings from the Health ABC study. Neurobiol. Aging. 2009;30:607–614. doi: 10.1016/j.neurobiolaging.2007.08.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Goumidi L., Dahlman-Wright K., Tapia-Paez I., Matsson H., Pasquier F., Amouyel P., Kere J., Lambert J.C., Meirhaeghe A. Study of estrogen receptor-alpha and receptor-beta gene polymorphisms on Alzheimer’s disease. J. Alzheimer’s Dis. Jad. 2011;26:431–439. doi: 10.3233/JAD-2011-110362. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Khorram Khorshid H.R., Gozalpour E., Saliminejad K., Karimloo M., Ohadi M., Kamali K. Vitamin D Receptor (VDR) polymorphisms and late-onset Alzheimer’s disease: An association study. Iran. J. Public Health. 2013;42:1253–1258. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Liu X., Jiao B., Shen L. The epigenetics of Alzheimer’s Disease: Factors and therapeutic implications. Front. Genet. 2018;9:579. doi: 10.3389/fgene.2018.00579. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Wainaina M.N., Chen Z., Zhong C. Environmental factors in the development and progression of late-onset Alzheimer’s disease. Neurosci. Bull. 2014;30:253–270. doi: 10.1007/s12264-013-1425-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Grant W.B., Campbell A., Itzhaki R.F., Savory J. The significance of environmental factors in the etiology of Alzheimer’s disease. J. Alzheimer’s Dis. Jad. 2002;4:179–189. doi: 10.3233/JAD-2002-4308. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Moulton P.V., Yang W. Air pollution, oxidative stress, and Alzheimer’s disease. J. Environ. Public Health. 2012;2012:472751. doi: 10.1155/2012/472751. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Croze M.L., Zimmer L. Ozone atmospheric pollution and Alzheimer’s disease: From epidemiological facts to molecular mechanisms. J. Alzheimer’s Dis. Jad. 2018;62:503–522. doi: 10.3233/JAD-170857. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Hu N., Yu J.T., Tan L., Wang Y.L., Sun L., Tan L. Nutrition and the risk of Alzheimer’s disease. Biomed. Res. Int. 2013;2013:524820. doi: 10.1155/2013/524820. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Abate G., Marziano M., Rungratanawanich W., Memo M., Uberti D. Nutrition and AGE-ing: Focusing on Alzheimer’s disease. Oxidative Med. Cell. Longev. 2017;2017:7039816. doi: 10.1155/2017/7039816. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Koyama A., Hashimoto M., Tanaka H., Fujise N., Matsushita M., Miyagawa Y., Hatada Y., Fukuhara R., Hasegawa N., Todani S., et al. Malnutrition in Alzheimer’s disease, dementia with lewy bodies, and frontotemporal lobar degeneration: Comparison using serum albumin, total protein, and hemoglobin level. PLoS ONE. 2016;11:e0157053. doi: 10.1371/journal.pone.0157053. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Adlard P.A., Bush A.I. Metals and Alzheimer’s disease. J. Alzheimer’s Dis. Jad. 2006;10:145–163. doi: 10.3233/JAD-2006-102-303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Colomina M.T., Peris-Sampedro F. Aluminum and Alzheimer’s disease. Adv. Neurobiol. 2017;18:183–197. doi: 10.1007/978-3-319-60189-2_9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 84. Huat T.J., Camats-Perna J., Newcombe E.A., Valmas N., Kitazawa M., Medeiros R. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J. Mol. Biol. 2019;431:1843–1868. doi: 10.1016/j.jmb.2019.01.018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Sochocka M., Zwolinska K., Leszek J. The infectious etiology of Alzheimer’s disease. Curr. Neuropharmacol. 2017;15:996–1009. doi: 10.2174/1570159X15666170313122937. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Fulop T., Itzhaki R.F., Balin B.J., Miklossy J., Barron A.E. Role of microbes in the development of Alzheimer’s disease: State of the art—An international symposium presented at the 2017 IAGG congress in San Francisco. Front. Genet. 2018;9:362. doi: 10.3389/fgene.2018.00362. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Muzambi R., Bhaskaran K., Brayne C., Smeeth L., Warren-Gash C. Common bacterial infections and risk of incident cognitive decline or dementia: A systematic review protocol. BMJ Open. 2019;9:e030874. doi: 10.1136/bmjopen-2019-030874. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Stampfer M.J. Cardiovascular disease and Alzheimer’s disease: Common links. J. Intern. Med. 2006;260:211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Santos C.Y., Snyder P.J., Wu W.C., Zhang M., Echeverria A., Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement. 2017;7:69–87. doi: 10.1016/j.dadm.2017.01.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. De Bruijn R.F., Ikram M.A. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130. doi: 10.1186/s12916-014-0130-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Alford S., Patel D., Perakakis N., Mantzoros C.S. Obesity as a risk factor for Alzheimer’s disease: Weighing the evidence. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018;19:269–280. doi: 10.1111/obr.12629. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Pegueroles J., Jimenez A., Vilaplana E., Montal V., Carmona-Iragui M., Pane A., Alcolea D., Videla L., Casajoana A., Clarimon J., et al. Obesity and Alzheimer’s disease, does the obesity paradox really exist? A magnetic resonance imaging study. Oncotarget. 2018;9:34691–34698. doi: 10.18632/oncotarget.26162. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Anjum I., Fayyaz M., Wajid A., Sohail W., Ali A. Does obesity increase the risk of dementia: A literature review. Cureus. 2018;10:e2660. doi: 10.7759/cureus.2660. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Lee H.J., Seo H.I., Cha H.Y., Yang Y.J., Kwon S.H., Yang S.J. Diabetes and Alzheimer’s disease: Mechanisms and nutritional aspects. Clin. Nutr. Res. 2018;7:229–240. doi: 10.7762/cnr.2018.7.4.229. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Singh R., Sadiq N.M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. [(accessed on 8 December 2020)]. Cholinesterase Inhibitors. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544336/ [ Google Scholar ]
- 96. Eldufani J., Blaise G. The role of acetylcholinesterase inhibitors such as neostigmine and rivastigmine on chronic pain and cognitive function in aging: A review of recent clinical applications. Alzheimers Dement. 2019;5:175–183. doi: 10.1016/j.trci.2019.03.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review) Mol. Med. Rep. 2019;20:1479–1487. doi: 10.3892/mmr.2019.10374. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Wang R., Reddy P.H. Role of glutamate and NMDA receptors in Alzheimer’s disease. J. Alzheimer’s Dis. Jad. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Kuns B., Rosani A., Varghese D. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. [(accessed on 8 December 2020)]. Memantine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK500025/ [ Google Scholar ]
- 100. Briggs R., Kennelly S.P., O’Neill D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016;16:247–253. doi: 10.7861/clinmedicine.16-3-247. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Crous-Bou M., Minguillon C., Gramunt N., Molinuevo J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer’s Res. Ther. 2017;9:71. doi: 10.1186/s13195-017-0297-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Crismon M.L. Tacrine: First drug approved for Alzheimer’s disease. Ann. Pharmacother. 1994;28:744–751. doi: 10.1177/106002809402800612. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Qizilbash N., Birks J., Lopez Arrieta J., Lewington S., Szeto S. Tacrine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2000:CD000202. doi: 10.1002/14651858.CD000202. [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Cacabelos R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr. Dis. Treat. 2007;3:303–333. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Kumar A., Sharma S. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. [(accessed on 8 December 2020)]. Donepezil. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513257/ [ Google Scholar ]
- 106. Dooley M., Lamb H.M. Donepezil: A review of its use in Alzheimer’s disease. Drugs Aging. 2000;16:199–226. doi: 10.2165/00002512-200016030-00005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Annicchiarico R., Federici A., Pettenati C., Caltagirone C. Rivastigmine in Alzheimer’s disease: Cognitive function and quality of life. Ther. Clin. Risk Manag. 2007;3:1113–1123. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Muller T. Rivastigmine in the treatment of patients with Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2007;3:211–218. doi: 10.2147/nedt.2007.3.2.211. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Khoury R., Rajamanickam J., Grossberg G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug Saf. 2018;9:171–178. doi: 10.1177/2042098617750555. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 110. Birks J., Grimley Evans J., Iakovidou V., Tsolaki M., Holt F.E. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2009:CD001191. doi: 10.1002/14651858.CD001191.pub2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Scott L.J., Goa K.L. Galantamine: A review of its use in Alzheimer’s disease. Drugs. 2000;60:1095–1122. doi: 10.2165/00003495-200060050-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Prvulovic D., Hampel H., Pantel J. Galantamine for Alzheimer’s disease. Expert Opin. Drug Metab. Toxicol. 2010;6:345–354. doi: 10.1517/17425251003592137. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Kim J.K., Park S.U. Pharmacological aspects of galantamine for the treatment of Alzheimer’s disease. Excli J. 2017;16:35–39. doi: 10.17179/excli2016-820. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 114. Wahba S.M., Darwish A.S., Kamal S.M. Ceria-containing uncoated and coated hydroxyapatite-based galantamine nanocomposites for formidable treatment of Alzheimer’s disease in ovariectomized albino-rat model. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;65:151–163. doi: 10.1016/j.msec.2016.04.041. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Liu J., Chang L., Song Y., Li H., Wu Y. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci. 2019;13:43. doi: 10.3389/fnins.2019.00043. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Huang Y.J., Lin C.H., Lane H.Y., Tsai G.E. NMDA Neurotransmission dysfunction in behavioral and psychological symptoms of Alzheimer’s disease. Curr. Neuropharmacol. 2012;10:272–285. doi: 10.2174/157015912803217288. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 117. Companys-Alemany J., Turcu A.L., Bellver-Sanchis A., Loza M.I., Brea J.M., Canudas A.M., Leiva R., Vazquez S., Pallas M., Grinan-Ferre C. A novel NMDA receptor antagonist protects against cognitive decline presented by senescent mice. Pharmaceutics. 2020;12:284. doi: 10.3390/pharmaceutics12030284. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 118. Folch J., Busquets O., Ettcheto M., Sanchez-Lopez E., Castro-Torres R.D., Verdaguer E., Garcia M.L., Olloquequi J., Casadesus G., Beas-Zarate C., et al. Memantine for the treatment of dementia: A Review on its current and future applications. J. Alzheimer’s Dis. Jad. 2018;62:1223–1240. doi: 10.3233/JAD-170672. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 119. Cummings J., Fox N. Defining disease modifying therapy for Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2017;4:109–115. doi: 10.14283/jpad.2017.12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 120. Huang L.K., Chao S.P., Hu C.J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020;27:18. doi: 10.1186/s12929-019-0609-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Neumann U., Ufer M., Jacobson L.H., Rouzade-Dominguez M.L., Huledal G., Kolly C., Luond R.M., Machauer R., Veenstra S.J., Hurth K., et al. The BACE-1 inhibitor CNP520 for prevention trials in Alzheimer’s disease. Embo Mol. Med. 2018;10:e9316. doi: 10.15252/emmm.201809316. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 122. Vandenberghe R., Riviere M.E., Caputo A., Sovago J., Maguire R.P., Farlow M., Marotta G., Sanchez-Valle R., Scheltens P., Ryan J.M., et al. Active Abeta immunotherapy CAD106 in Alzheimer’s disease: A phase 2b study. Alzheimers Dement. 2017;3:10–22. doi: 10.1016/j.trci.2016.12.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 123. Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement. 2020;6:e12050. doi: 10.1002/trc2.12050. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 124. Tolar M., Abushakra S., Hey J.A., Porsteinsson A., Sabbagh M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer’s Res. Ther. 2020;12:95. doi: 10.1186/s13195-020-00663-w. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 125. Galimberti D., Scarpini E. Disease-modifying treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2011;4:203–216. doi: 10.1177/1756285611404470. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 126. Ghezzi L., Scarpini E., Galimberti D. Disease-modifying drugs in Alzheimer’s disease. Drug Des. Dev. Ther. 2013;7:1471–1478. doi: 10.2147/DDDT.S41431. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 127. Medina M. An Overview on the clinical development of tau-based therapeutics. Int. J. Mol. Sci. 2018;19:1160. doi: 10.3390/ijms19041160. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 128. Davidowitz E.J., Krishnamurthy P.K., Lopez P., Jimenez H., Adrien L., Davies P., Moe J.G. In vivo validation of a small molecule inhibitor of tau self-association in htau mice. J. Alzheimer’s Dis. Jad. 2020;73:147–161. doi: 10.3233/JAD-190465. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 129. Cortez L., Sim V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion. 2014;8:197–202. doi: 10.4161/pri.28938. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 130. Campanella C., Pace A., Caruso Bavisotto C., Marzullo P., Marino Gammazza A., Buscemi S., Palumbo Piccionello A. Heat shock proteins in Alzheimer’s disease: Role and targeting. Int. J. Mol. Sci. 2018;19:2603. doi: 10.3390/ijms19092603. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 131. Wilhelmus M.M., de Waal R.M., Verbeek M.M. Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer’s disease. Mol. Neurobiol. 2007;35:203–216. doi: 10.1007/s12035-007-0029-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 132. Martin-Pena A., Rincon-Limas D.E., Fernandez-Funez P. Engineered Hsp70 chaperones prevent Abeta42-induced memory impairments in a Drosophila model of Alzheimer’s disease. Sci. Rep. 2018;8:9915. doi: 10.1038/s41598-018-28341-w. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 133. Calderwood S.K., Murshid A. Molecular chaperone accumulation in cancer and decrease in Alzheimer’s disease: The potential roles of HSF1. Front. Neurosci. 2017;11:192. doi: 10.3389/fnins.2017.00192. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 134. Repalli J., Meruelo D. Screening strategies to identify HSP70 modulators to treat Alzheimer’s disease. Drug Des. Dev. Ther. 2015;9:321–331. doi: 10.2147/DDDT.S72165. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 135. Li X., Shao H., Taylor I.R., Gestwicki J.E. Targeting allosteric control mechanisms in heat shock protein 70 (Hsp70) Curr. Top. Med. Chem. 2016;16:2729–2740. doi: 10.2174/1568026616666160413140911. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 136. Abisambra J., Jinwal U.K., Miyata Y., Rogers J., Blair L., Li X., Seguin S.P., Wang L., Jin Y., Bacon J., et al. Allosteric heat shock protein 70 inhibitors rapidly rescue synaptic plasticity deficits by reducing aberrant tau. Biol. Psychiatry. 2013;74:367–374. doi: 10.1016/j.biopsych.2013.02.027. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 137. Bohush A., Bieganowski P., Filipek A. Hsp90 and its co-chaperones in neurodegenerative diseases. Int. J. Mol. Sci. 2019;20:4976. doi: 10.3390/ijms20204976. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. Ou J.R., Tan M.S., Xie A.M., Yu J.T., Tan L. Heat shock protein 90 in Alzheimer’s disease. Biomed Res. Int. 2014;2014:796869. doi: 10.1155/2014/796869. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 139. Wang B., Liu Y., Huang L., Chen J., Li J.J., Wang R., Kim E., Chen Y., Justicia C., Sakata K., et al. A CNS-permeable Hsp90 inhibitor rescues synaptic dysfunction and memory loss in APP-overexpressing Alzheimer’s mouse model via an HSF1-mediated mechanism. Mol. Psychiatry. 2017;22:990–1001. doi: 10.1038/mp.2016.104. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 140. Li J.G., Chiu J., Ramanjulu M., Blass B.E., Pratico D. A pharmacological chaperone improves memory by reducing Abeta and tau neuropathology in a mouse model with plaques and tangles. Mol. Neurodegener. 2020;15:1. doi: 10.1186/s13024-019-0350-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 141. Vagnozzi A.N., Li J.G., Chiu J., Razmpour R., Warfield R., Ramirez S.H., Pratico D. VPS35 regulates tau phosphorylation and neuropathology in tauopathy. Mol. Psychiatry. 2019 doi: 10.1038/s41380-019-0453-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] [ Retracted ]
- 142. Li J.G., Chiu J., Pratico D. Full recovery of the Alzheimer’s disease phenotype by gain of function of vacuolar protein sorting 35. Mol. Psychiatry. 2020;25:2630–2640. doi: 10.1038/s41380-019-0364-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] [ Retracted ]
- 143. Mecozzi V.J., Berman D.E., Simoes S., Vetanovetz C., Awal M.R., Patel V.M., Schneider R.T., Petsko G.A., Ringe D., Small S.A. Pharmacological chaperones stabilize retromer to limit APP processing. Nat. Chem. Biol. 2014;10:443–449. doi: 10.1038/nchembio.1508. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 144. Knight E.M., Williams H.N., Stevens A.C., Kim S.H., Kottwitz J.C., Morant A.D., Steele J.W., Klein W.L., Yanagisawa K., Boyd R.E., et al. Evidence that small molecule enhancement of beta-hexosaminidase activity corrects the behavioral phenotype in Dutch APP(E693Q) mice through reduction of ganglioside-bound Abeta. Mol. Psychiatry. 2015;20:109–117. doi: 10.1038/mp.2014.135. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 145. Andrade S., Ramalho M.J., Loureiro J.A., Pereira M.D.C. Natural compounds for Alzheimer’s disease therapy: A systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 2019;20:2313. doi: 10.3390/ijms20092313. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Ma Y., Yang M.W., Li X.W., Yue J.W., Chen J.Z., Yang M.W., Huang X., Zhu L.L., Hong F.F., Yang S.L. Therapeutic effects of natural drugs on Alzheimer’s disease. Front. Pharmacol. 2019;10:1355. doi: 10.3389/fphar.2019.01355. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.4 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 23 August 2024
Recent advances in Alzheimer’s disease: mechanisms, clinical trials and new drug development strategies
- Jifa Zhang 1 na1 ,
- Yinglu Zhang 1 na1 ,
- Jiaxing Wang ORCID: orcid.org/0000-0002-3202-2402 2 na1 ,
- Yilin Xia 1 ,
- Jiaxian Zhang 1 &
- Lei Chen 1
Signal Transduction and Targeted Therapy volume 9 , Article number: 211 ( 2024 ) Cite this article
21k Accesses
13 Citations
19 Altmetric
Metrics details
- Drug discovery
- Medicinal chemistry
Alzheimer’s disease (AD) stands as the predominant form of dementia, presenting significant and escalating global challenges. Its etiology is intricate and diverse, stemming from a combination of factors such as aging, genetics, and environment. Our current understanding of AD pathologies involves various hypotheses, such as the cholinergic, amyloid, tau protein, inflammatory, oxidative stress, metal ion, glutamate excitotoxicity, microbiota-gut-brain axis, and abnormal autophagy. Nonetheless, unraveling the interplay among these pathological aspects and pinpointing the primary initiators of AD require further elucidation and validation. In the past decades, most clinical drugs have been discontinued due to limited effectiveness or adverse effects. Presently, available drugs primarily offer symptomatic relief and often accompanied by undesirable side effects. However, recent approvals of aducanumab ( 1 ) and lecanemab ( 2 ) by the Food and Drug Administration (FDA) present the potential in disrease-modifying effects. Nevertheless, the long-term efficacy and safety of these drugs need further validation. Consequently, the quest for safer and more effective AD drugs persists as a formidable and pressing task. This review discusses the current understanding of AD pathogenesis, advances in diagnostic biomarkers, the latest updates of clinical trials, and emerging technologies for AD drug development. We highlight recent progress in the discovery of selective inhibitors, dual-target inhibitors, allosteric modulators, covalent inhibitors, proteolysis-targeting chimeras (PROTACs), and protein-protein interaction (PPI) modulators. Our goal is to provide insights into the prospective development and clinical application of novel AD drugs.
Similar content being viewed by others

New therapeutics beyond amyloid-β and tau for the treatment of Alzheimer’s disease
DUBs in Alzheimer’s disease: mechanisms and therapeutic implications
Emerging diagnostics and therapeutics for Alzheimer disease
Introduction.
Dementia has emerged as a global health challenge. According to the World Health Organization’s 2022 blueprint for dementia research, an estimated 55.2 million individuals globally are affected. The prevalence among those over the age of 60 varies by region: with Southeast Asia reporting a prevalence of 2.9%, Europe at 6.5%, and other regions experiencing rates between 3.1% and 5.7%. 1 The incidence of dementia is generally increasing, while some high-income countries are seeing a decline. 2 By 2030, the estimated number of people living with dementia will surge to 78 million. Furthermore, the global financial burden associated with medical care, social services, and informal caregiving for those with dementia is expected to exceed US$ 2.8 trillion. This situation will have a profound impact on individuals, families, and societies. 1 Alzheimer’s disease (AD), the predominant form of dementia, exhibits similar epidemiological trends and represents an urgent and escalating challenge worldwide. In the United States, approximately one in nine individuals (10.8%) age 65 and older suffer from AD, with an annual incidence of 1275 new cases per 100,000 persons. 3 , 4 Patients with AD exhibit a substantial accumulation of amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs) within their brains, accompanied by a cascade of pathological processes such as neuroinflammation, synaptic dysfunction, mitochondrial and bioenergetic disturbances, as well as vascular abnormalities. Collectively these processes may ultimately lead to the death of neurons. 5 , 6 Clinically, the primary hallmark of AD is amnestic cognitive impairment. Initially, symptoms may manifest as depression, anxiety, social withdrawal, and altered sleep patterns. As the disease progresses, symptoms worsen, leading to severe memory loss, neuropsychiatric symptoms such as hallucinations and delusions, and intensified behavioral and emotional issues in its advanced stages. Additionally, some patients with non-amnestic cognitive impairment may experience varying levels of dysfunctions in visual-spatial, language, executive functions, behavior, or motor skills. 2 , 7 , 8 , 9 Moreover, comorbidities linked with AD may exacerbate the health condition of patients, contributing to clinical phenotype diversity and accelerating cognitive dysfunction. Such conditions include hypercholesterolemia, hypertension, diabetes, obesity, depression, and cardiovascular diseases. Additionally, complications arising from AD progressions, like thrombosis, mobility impairments, dysphagia, malnutrition, and pneumonia (lung infections), may considerably diminish the life quality of patients and increase mortality risk. 2 , 4 , 10 , 11 , 12 , 13 , 14 The connection between comorbidities and the pathological changes in AD is currently the subject of ongoing research. 15 , 16 , 17 Unfortunately, there is yet no cure for AD, and patients are frequently diagnosed at a late and irreversible stage, facing an average survival period of 4–8 years. 4 , 18 , 19 Nonetheless, pathological changes in the brain begin during the preclinical stage, decades before clinical symptoms. Typically, patients transit to mild cognitive impairment (MCI) around 6-10 years later, with approximately 15% progressing to AD within 2 years and one-third within 5 years. 4 , 20 , 21 Therefore, it’s crucial to concentrate on the preclinical and MCI stages, where early intervention and management of modifiable risk factors could potentially lower the risk of onset or delay the progression of disease. 22 Evidence suggests that about one-third of AD cases worldwide are closely linked to modifiable risk factors. 23 Encouragingly, due to improvements in risk factors such as vascular health, lifestyle choices, and education levels, the incidence of AD is on a downward trend in the United States, South Korea, Europe, and certain regions of Asia. 2 , 24 In recent years, numerous articles 4 , 22 , 23 , 25 , 26 , 27 , 28 have highlighted modifiable risk factors for AD, alongside the benefits of Multidomain Alzheimer Preventive Trials. These insights underscore the efficacy of early prevention strategies for AD.
The etiology of AD is complex and diverse, and the precise mechanisms underlying its onset are not yet completely understood. Beyond the pivotal role of Aβ and tau, a spectrum of other factors may contribute to the pathology of AD, such as acetylcholine deficiency, neuroinflammation, oxidative stress, biometal dyshomeostasis, glutamate imbalance, insulin resistance, gut microbiome abnormalities, cholesterol homeostasis disruption, mitochondrial dysfunction, and autophagy abnormalities 29 , 30 , 31 (Fig. 1 ). Of note, these factors also form the foundation for clinical diagnosis and treatment strategies. Biomarkers can identify patients in the early stages, monitor disease progression, and evaluate the effectiveness of drugs. 32 , 33 , 34 , 35 The hypotheses surrounding these pathogenic factors provide potential targets for drug development. However, the development of effective AD drugs has been fraught with challenges. Tacrine ( 3 ) 36 , 37 , 38 , 39 , 40 was withdrawn from the market primarily because of its hepatotoxicity. Medications such as donepezil ( 4 ), 41 , 42 , 43 rivastigmine ( 5 ), 44 , 45 galantamine ( 6 ), 46 , 47 , 48 memantine ( 7 ), 49 , 50 and namzaric ( 8 ) 51 , 52 have been employed in clinical settings. While these drugs can temporarily alleviate or stabilize symptoms, they are unable to stop the long-term progression of the disease and are associated with various side effects. 33 , 53 New drugs, including sodium oligomannate ( 9 , GV-971), 54 , 55 , 56 aducanumab ( 1 ), 57 , 58 , 59 lecanemab ( 2 ), 60 , 61 , 62 and donanemab ( 10 , currently under review for market approval), 63 which aim to offer disease-modifying therapies that intervene in the progression of AD. Their clinical relevance remains to be evaluated thoroughly. More than a century has elapsed since AD was first described in 1906, 64 and significant progress has been made in understanding its pathogenesis, improving diagnosis, and enhancing treatment. 65 , 66 Unfortunately, the current offerings fall short of meeting the need to address cognitive. Therefore, this review takes into account the AD research framework of prevention, diagnosis, and treatment, and discusses the pathogenesis, diagnostic biomarkers, clinical trials, and next-generation small molecule drugs. It also emphasizes the critical need to improve the safety and efficacy of drugs through innovative drug development techniques, such as selective inhibitors, 67 dual-target inhibitors, 68 , 69 allosteric modulators, 70 , 71 covalent inhibitors, 72 proteolysis-targeting chimeras (PROTACs) 73 and protein-protein interaction (PPI) modulators, 74 , 75 aiming for more effective clinical translation from outcomes of research.

Diagram for the pathogenesis of AD, including the cholinergic hypothesis, 619 , 620 the glutamatergic hypothesis, 621 the amyloid hypothesis, 622 , 623 the tau protein hypothesis, 624 , 625 the inflammatory hypothesis, 626 , 627 the microbiota-gut-brain axis hypothesis, 628 , 629 the oxidative stress hypothesis, 191 the metal ion hypothesis, 630 , 631 and the abnormal autophagy hypothesis 235
Mechanisms of AD
Numerous hypotheses have been proposed to unravel the pathogenesis of AD, yet a unified theory remains elusive, likely due to the complex nature of AD. AD can be categorized into two main types: familial (accounting for 1-5% of AD cases) and sporadic forms (over 95% of cases). 76 Familial AD (FAD) is predominantly characterized by autosomal dominant genetic mutations in amyloid precursor protein (APP), presenilin 1 (PS1), and presenilin 2 (PS2) genes, typically manifesting between 30-65 years and progressing rapidly. In contrast, sporadic AD (SAD), also known as late-onset AD, usually manifests after the age of 65 and is influenced by a combination of genetic risks, environmental factors, and various comorbidities. 77 , 78 , 79 Genome-wide association studies (GWAS) and genome-wide meta-analyses have identified numerous genetic risk loci associated with SAD, implicating pathways in immune response, lipid metabolism, Aβ plaque, NFTs, and endocytosis, yet many loci remain undiscovered. 80 , 81 , 82 , 83 Non-genetic factors such as lifestyles, psychosocial factors, environment, and diseases related to AD (comorbidities and complications), may elevate the risk of developing AD. They may achieve this by altering biological pathways and genetic susceptibility, 23 , 84 , 85 , 86 making it challenging to pinpoint a direct cause of clinical pathology in AD. Furthermore, different AD subtypes (typical and atypical) often exhibit various clinical symptoms. 87 , 88 , 89 Thirdly, AD has multiple pathological features including Aβ plaques, NFTs, synaptic and neuronal loss, and neuroinflammation. 90 , 91 Overall, the diversity of triggers, clinical manifestations, and neuropathological features underlie the heterogeneity of AD. Consequently, developing a comprehensive theoretical framework that links genetic foundations, molecular mechanisms, and clinical phenotypes of AD is extremely challenging. Current limitations in AD research also hinder our comprehensive understanding of its pathophysiology. 1 Moreover, the high failure rate of clinical trials makes it difficult to effectively validate hypotheses, possibly attributed to the coexistence of multiple theories (which will be detailed in subsequent sections).
Cholinergic hypothesis
The cholinergic hypothesis was the earliest to delineate the pathogenesis of AD. It describes the severe damage of cholinergic neurons in the nucleus basalis of meynert (NBM), leading to a marked decrease in choline acetyltransferase (ChAT) activity within the primary projection areas - the cerebral cortex and hippocampus (regions associated with learning and memory). Additionally, this neuronal damage is accompanied by a significant increase in the density of senile plaques. The scenario in the cholinergic hypothesis suggests a close relationship between deficits of basal forebrain cholinergic and cognitive impairments observed in AD. 91 , 92 , 93 , 94 , 95 , 96 , 97 Cholinergic neurons in the basal forebrain are crucial components of the central cholinergic system, significant contributing to the regulation of cognitive functions, attention, and memory. 98 These cell bodies of neurons are predominantly located in the medial septal nucleus (MSN), diagonal band of broca (DBB), NBM, and substantia innominata (SI). 97 , 99 It has been observed that cholinergic neurons in the NBM region are particularly susceptible to degeneration and loss in AD. It is believed to be associated with nerve growth factor (NGF)-dependent nutritional depletion. 100 , 101 Acetylcholine (ACh) is synthesized from choline and acetyl-coenzyme A by ChAT, then transported into synaptic vesicles through the vesicular acetylcholine transporter (VAChT). When a neural signal arrives, ACh is released, where it binds to muscarinic and nicotinic acetylcholine receptors (mAChRs and nAChRs) on the postsynaptic membrane to transmit neural signals. Subsequently, ACh in the synaptic cleft is degraded into choline by acetylcholinesterase (AChE) and reabsorbed into presynaptic cholinergic neurons. 31 , 102 , 103 , 104 The decline in the activity of ChAT, combined with the detrimental effects of Aβ on nutritional imbalance, the synthesis, release, and degradation of ACh, leads to a reduction of ACh levels. This decrease impairs its physiological functions in learning, memory, motor regulation, and sleep cycle regulation. 97 , 105 , 106 , 107 , 108 In summary, the cholinergic hypothesis, as a well-established and classic theory, has significantly advanced the early research and drug development for AD. AChE inhibitors (AChEIs), like donepezil ( 4 ), rivastigmine ( 5 ), and galantamine ( 6 ), which are approved over two decades ago, remain the mainstay of AD treatment in clinical management. 109 Despite these advancements, the limited efficacy and side effects of such drugs, coupled with the presence of non-cholinergic groups in AD, 99 and non-specificity in these pathological features, 94 challenge the cholinergic hypothesis to fully explain the complex of AD pathology.
Amyloid hypothesis
The accumulation of Aβ is a hallmark pathological feature in both extensively studied autosomal dominant AD and sporadic late-onset AD patients. 110 Aβ originates from the processing of the APP, a transmembrane glycoprotein, through its sequential cleavage by β-secretase and γ-secretase (a multiprotein complex with PS1 or PS2 as catalytic subunits). This process yields various lengths of Aβ fragments, with Aβ 40 and Aβ 42 being the predominant. The hydrophobic C-terminal of Aβ 42 facilitates the β-sheet conformational transition and the aggregation and formation of the core component of senile plaques. 78 , 111 , 112 Mutations in PS1, a typical mutation in FAD, potentially promote Aβ accumulation through multiple mechanisms, including increased Aβ production and impairment of autophagy functions. 83 , 113 , 114 , 115 However, FAD mutations are not necessarily linked to an increase in Aβ 42 levels or an elevation of Aβ 42 /Aβ 40 ratio. 78 , 116 The plaque formation in SAD is notably more intricate, related to a dynamic imbalance between Aβ production and clearance mechanisms. 117 Apolipoprotein E (APOE), particularly the ε4 allele, stands out as the most crucial genetic risk factor for SAD. Carrying one or two APOE ε4 alleles increases the risk of AD by 2-3 and 12-fold, respectively. 118 Research indicates that APOE protein is detectable in neuritic plaques, and individuals with the APOEε4 allele also have a higher burden of Aβ plaques in their brains, 119 , 120 highlighting its critical influences on Aβ deposition. While the exact mechanisms remain to be agreed upon, both in vitro and in vivo experiments suggested several potential pathways for APOEε4, including enhancing Aβ production (promoting APP transcription and processing), facilitating Aβ aggregation (interaction with soluble and fibrillary Aβ aids in seeding/oligomerization/protofibril formation), and impairing Aβ clearance (disrupted glial and enzymatic Aβ degradation functions, and Aβ removal rate from the brain). 121 , 122 , 123 , 124 Moreover, other genetic risk factors, 125 , 126 cardiovascular health issues (such as diabetes, hypercholesterolemia), and lifestyle factors (such as diet and sleep) 127 have also been extensively studied in recent years for their relationship with Aβ metabolism in SAD. The toxicity mechanism of Aβ aggregates remains uncertain, but different perspectives exist: 77 , 128 Aβ might cause AD pathology through the loss of physiological functions during the aggregation process. Aβ monomers have neuroprotective properties, with assumed roles in antioxidant and antimicrobial activities, improving the condition of damaged nervous systems, regulating the vascular system, and enhancing synaptic plasticity. 129 , 130 Soluble Aβ oligomers are the primary neurotoxic substances, 131 , 132 , 133 disruption of cell membrane integrity, 134 activation in inflammatory responses, 135 , 136 causes of calcium homeostasis imbalance 137 and mitochondrial dysfunction, 138 , 139 , 140 triggers in oxidative stress, 141 and damage factor of synapses. 142 The potential downstream pathways of oligomers on neurons and glial cells are illustrated in Fig. 2 and Fig. 3 . The amyloid cascade 143 has been proposed for over 30 years, which provided crucial insights into the mechanisms of AD’s onset and progression. This hypothesis has led to the development of drugs, including β-secretase inhibitors, γ-secretase inhibitors and modulators, anti-amyloid antibodies, Aβ vaccine, and Aβ aggregation inhibitors, aimed at delaying the disease’s advancement. Currently, antibodies like aducanumab ( 1 ), lecanemab ( 2 ), and donanemab ( 10 ) show their promise in proving Aβ as a significant factor in AD development. However, in light of beneficial effects on reducing Aβ brain burden, the clinical value of these drugs remains to be validated. 77 , 78 Of note, the amyloid cascade hypothesis remains controversial. This theory faces challenges in explaining the diverse pathological features, shows a weak correlation between Aβ and cognitive decline, and has failed to demonstrate efficacy in numerous clinical drugs to target Aβ. 118 , 144 , 145 , 146 , 147 These findings suggest that Aβ deposition or plaque formation might not be the actual cause of the disease, but rather a result or secondary factor of the pathological process. 77 , 148 Given the increasingly recognized critical role of tau, the pathological sequence and interplay of tau and Aβ in AD deserve further exploration. 149 , 150 , 151
Schematic illustration depicting the possible molecular downstream pathways of Aβ on neuronal synapses and astrocytes. (1) Aβ is capable of interacting with cell membranes and binding to a variety of synaptic receptors such as PrP C , NMDA receptors, P75 NTR , and mGluR5, which leads to a cascade of events including calcium dyshomeostasis, inhibition of long-term potentiation (LTP), tau hyperphosphorylation, mitochondrial dysfunction, and oxidative stress, ultimately resulting in neuronal death. 112 , 632 , 633 (2) Aβ blocks the reuptake of glutamate by excitatory amino acid transporter (EAAT) receptors, causing glutamate accumulation intersynaptically and neuronal hyperactivity. 634 (3) Aβ and some pro-inflammatory cytokines (such as TNFα, IL-1α, and C1q) may induce the A1 phenotype of astrocytes. This transformation may involve altering astrocyte functions and modulating their interactions with other cells (such as neurons and microglia), thereby participating in processes such as Aβ deposition, neuroinflammation, synaptic loss, and neuronal death. 635 , 636 , 637 (4) APOE, primarily released from astrocytes, associates with lipoproteins to form APOE-associated lipoprotein particles, which can bind to soluble Aβ and mediate its clearance 119

Schematic illustration depicting potential molecular downstream pathways of Aβ on microglia. Microglia has numerous pattern recognition receptors that can bind to Aβ, initiating an inflammatory cascade. This process promotes the assembly and activation of NLRP3, leading to the release of pro-inflammatory cytokines, which further exacerbate the aggregation of Aβ. 171 In addition, the diagram also encompasses the downstream signaling pathways of TREM2. 638 , 639 Some variants associated with AD, such as the TREM2 variant R47H, may potentially diminish the binding or internalization of TREM2 with ligands such as APOE-Aβ complexes, APOE, phospholipids, and Aβ. This reduction may consequently impair the activation of microglial cells, thereby compromising their ability to clear amyloid plaques. 638 , 640 , 641 , 642 , 643 It is worth noting that there remain many uncertainties and controversies regarding the in vivo ligands and signaling pathways of TREM2, as well as the relationship between TREM2 variants and AD. Future in vivo experiments are needed to elucidate these aspects

Tau protein hypothesis
As a major component of NFTs, tau protein exhibits a spatial and temporal distribution that strongly correlates with clinical symptoms, making it a highly specific pathological biomarker in AD patients. 152 Tau is a microtubule-associated protein predominantly expressed in the axons of neurons, with lower expression levels in dendrites, soma, and glial cells. 153 , 154 It hosts numerous phosphorylation sites across its N-terminal region, C-terminal region, and repeat region, which are regulated by a balance of various kinases and phosphatases to maintain normal neuronal physiological functions. 150 , 155 Under pathological conditions, an imbalanced activity of phosphatases and kinases leads to hyperphosphorylation of tau. 156 , 157 This process leads to the detachment of tau protein from microtubules, followed by conformational changes and mislocalization, accumulation of tau oligomers, paired helical filaments (PHFs), and NFTs within the cell body and dendrites. These changes ultimately impair neuronal function and cause cell death. 158 , 159 , 160 Additionally, other post-translational modifications, including truncation, 161 , 162 glycosylation, 163 glycation, 164 and sumoylation, 165 play an active role in promoting tau aggregation and increasing its toxicity. Tau oligomers not only generate neurotoxicity within cells but also facilitate pathological spread through synaptic transmission. This process induces the aggregation of monomeric tau in recipient neurons, leading to the formation of new oligomers. 166 Overall, the significance of tau in AD pathogenesis stems from the strong correlation between tau accumulation and cognitive symptoms. 152 In recent years, there has been a heightened focus on tau deposition, including the correlation between tau deposition, brain atrophy, and glucose metabolism in both typical and atypical AD, 167 , 168 as well as the effects of tau deposition at the molecular and cellular levels. 169 Despite initial investigations into drugs based on the tau hypothesis not yielding promising results, 152 numerous treatments are still actively being developed. These include kinase inhibitors, tau aggregation inhibitors, tau immunotherapies, antisense oligonucleotides that inhibit tau production, agents that promote autophagy-mediated degradation, and tau-targeted PROTACs. 166 , 170
Neuroinflammation hypothesis
Neuroinflammation is generally characterized as a chronic inflammatory response in the central nervous system (CNS) that fails to resolve on its own. It often involves the activation of glial cells and the release of pro-inflammatory factors during neuroinflammation. 171 Microglia, the CNS foremost innate immune cells, acts as an initial defense against danger-associated molecular patterns and pathogen-associated molecular pattern receptors. Microglia are elongated, branched cells that monitor their environment and secrete neurotrophic factors in a state of homeostasis. Once stimulation is detected, microglia undergo morphological changes and initiate a variety of responses. 172 , 173 Aβ is a typical trigger for microglial activation. Activated microglia migrate towards senile plaques, engulf Aβ, and release enzymes to break down Aβ. Over prolonged periods, they might become less efficient at handling Aβ but continue to generate proinflammatory cytokines. 174 , 175 Aβ also causes the formation and activation of the NLRP3 inflammasome within microglia, which releases ASC specks that bind rapidly to Aβ in promoting Aβ aggregates and the spread of Aβ pathology. 176 Interactions between microglia and tau protein in the later stages of AD may contribute to increased tau phosphorylation and exosomal tau secretion, thereby promoting the spread of tau. 177 , 178 With the exaggerated activation, the complement cascade potentially leads to aberrant synapse pruning by microglia, further exacerbating AD pathology. 171 Researchers have identified different activation stages of microglia, each associated with distinct gene expression patterns. Initial stages were characterized by genes related to cell proliferation, whereas later stages feature genes linked to immune responses. 171 GWAS have pinpointed numerous risk genes closely linked to microglial activities, highlighting the significance of microglia as a promising therapeutic target. 179 Targeting triggering receptor expressed on myeloid cells 2 (TREM2) has the potential to harness neuroprotective properties by elevating microglial responsiveness to pathological proteins. 180 Meanwhile, APOE4 could modify the behavior and function of activated microglia, contributing to increased Aβ deposition, tau-associated neurodegeneration, enhanced inflammation, altered immune responses, and disrupted synaptic homeostasis. 123 , 181 , 182 , 183 , 184 Consequently, diminishing APOE4 expression in Aβ plaque-associated microglia may offer an effective approach. In summary, neuroinflammation is intricately associated with Aβ and tau pathologies, and the discovery of numerous immune response-related risk factors indicates that neuroinflammation is a significant factor in AD pathogenesis. Recent investigations have also expanded the scope of AD-related inflammation, exploring how the gut microbiota, oral microbiome, and viruses such as herpesviruses and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) impact neuroinflammation. 185 , 186 , 187 Regarding anti-inflammatory therapies, the effectiveness of nonsteroidal anti-inflammatory drugs (NSAIDs) remains inconclusive. 188 , 189 Despite this, the primary focuses in the development of anti-inflammatory drugs are appropriate intervention timing and enhancing target specificity. 171 , 190 Currently, numerous drugs targeting inflammation-related receptors, signaling pathways, and pro-inflammatory cytokines are under clinical trials. 185
Oxidative stress hypothesis
During regular metabolic processes, the body produces reactive oxygen species (ROS), reactive nitrogen species, and other highly reactive and unstable substances. These substances are generally kept at low levels by an efficient antioxidant defense system to protect cells from oxidative damage. 191 , 192 However, in the brain of AD patients, factors such as metal accumulation, overexpression of related enzymes (e.g., NADPH oxidase), and mitochondrial dysfunction are involved in producing excessive ROS, surpassing the ability of the endogenous antioxidant system and resulting in an oxidative imbalance. It will damage neuronal membrane lipids, proteins, and nucleic acids, ultimately causing neuronal cell death. 191 , 193 , 194 , 195 The abnormality of the electron transport chain within mitochondria is particularly a significant contributor to free radical production. Aβ plays a crucial role in mitochondrial dysfunction by reducing the activities of key enzymes and disrupting the dynamics of mitochondria. 192 , 196 Oxidative stress presented in the early stages of AD acts as a crosstalk between different hypotheses of AD. 197 For example, oxidative stress modulates the process of APP and the activity of secretases, thereby promoting the amyloid pathway. Furthermore, it is instrumental in the phosphorylation of tau proteins and the subsequent formation of NFTs. The activation of microglia induced by ROS triggers a neuroinflammatory cycle. The presence of free metals and complexes of Aβ with metals act as catalysts for ROS production, ultimately leading to neuronal cell death. 195 Given these connections between oxidative stress and other AD mechanisms, antioxidants have emerged as promising agents in AD treatment with positive outcomes observed in animal models. 198 However, the efficacy of antioxidants in clinical trials for AD remains uncertain. Several studies have indicated that standalone treatments or treatments in combination with cholinesterase inhibitors did not confer significant cognitive benefits to patients with AD. Future efforts should focus on optimizing drug dosages and initiating antioxidant therapy early in the course of the disease’s progression for potentially improved outcomes. 199 In summary, oxidative stress has garnered widespread attention as a significant factor in the pathogenesis of AD. Nevertheless, the interplay between Aβ and oxidative stress, 200 as well as their sequence within AD, 201 , 202 require further research and exploration.
Metal ion hypothesis
In physiological conditions, trace metals maintain homeostasis of the neuronal metal ion microenvironment. This balance can be disrupted by the inappropriate deposition or misdistribution of metal ions, with the dyshomeostasis of Fe 2+ , Cu 2+ , and Zn 2+ closely associated with AD. 203 The accumulation of these biometals in Aβ plaques and NFTs plays a critical role in pathological protein deposition. For instance, they may modulate the activity of essential enzymes, alter the conformation of proteins, or disrupt clearing pathways. 203 , 204 , 205 When metals are sequestered in protein deposits, it may initiate a cascade of ROS production and accentuate toxicity. 206 Specifically, iron-induced oxidative stress causes increased release of iron from iron-containing proteins, converting Fe 3+ to Fe 2+ intracellularly. Fe 2+ overload can induce ferroptosis and lipid peroxidation through the generation of ROS via the Fenton reaction, ultimately resulting in neuronal death. Similarly, Cu + directly binds to lipoylated dihydrolipoyl transacetylase (DLAT), inducing lipoylated DLAT aggregation and ultimately leading to cuproptosis. 203 The sequestration in protein deposits also causes functional metal loss, potentially contributing to the cognitive decline in AD. Zinc could interfere with signaling through N-methyl-D-aspartate (NMDA) receptors. Supplementation of zinc may promote the maturation of proBNDF, reducing synaptic dysfunction and neuronal death. 204 , 205 Hence, zinc deficiency is crucial in the context of glutamate excitotoxicity and synaptic dysfunction in AD. Overall, metal dyshomeostasis is closely linked to various events in AD such as amyloidosis, tauopathy, oxidative stress, and neuronal death. This hypothesis provides an alternative approach to understanding the pathogenesis of AD and detecting pathological changes. Further research is necessary to elucidate its role in AD. Additionally, metal ion chelators, developed based on this hypothesis, need to overcome challenges such as adverse events and poor blood-brain barrier (BBB) permeability to demonstrate their potential therapeutic value. 203
Glutamatergic excitotoxicity
Glutamate is the main excitatory neurotransmitter of glutamatergic neurotransmission in the CNS. 206 Their receptors comprise ionotropic glutamate receptors, including NMDA receptors, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, and kainate receptors, as well as metabotropic glutamate (mGlu) receptors. 207 Glutamate mainly interacts with NMDA receptors to control the influx of sodium and calcium to neurons. Magnesium ions act to shut the NMDA receptor’s cationic channel and block the entry of ions into neurons under physiological conditions. However, in AD, there is an overstimulation of NMDA receptors, which results in the dislodgement of magnesium and permits an excessive entry of sodium and calcium ions. 208 , 209 The entry of sodium into neurons causes their temporary swelling, while an increase in calcium levels initiates various Ca 2+ -dependent processes. These processes include the creation of ROS, disruption of mitochondrial function, and the activation of necrotic/apoptotic pathways, ultimately resulting in permanent excitotoxic damage to the neurons. 210 , 211 Overall, pharmaceutical validation of the glutamatergic excitotoxicity hypothesis demonstrates the effectiveness of neurotransmitter regulation in improving cognitive symptoms. However, the limitations of neurotransmitter-based medications and the focus on other hypotheses appear to hinder further investigation into the mechanisms of excitotoxicity. The observed changes in the inhibitory neurotransmitter system, exemplified by γ-aminobutyric acid, 212 and the potential for excitotoxicity to alter cognitive levels earlier than Aβ and tau pathologies, 209 suggest that excitotoxicity might hold greater potential in AD treatment.
Microbiota-gut-brain axis hypothesis
In recent years, the microbiota-gut-brain axis hypothesis has attracted significant attention, unveiling potential pathways for novel therapeutic strategies. 213 The microbiota predominantly consists of bacteria, with smaller populations of fungi, viruses, archaea, and protozoa. These microorganisms offer trophic and protective effects in metabolism and innate immunity and influence brain function via the gut-microbiota-brain axis. 214 , 215 , 216 The microbiota-gut-brain axis refers to a bidirectional communication system between the gut and the brain, including metabolic, endocrine, neural, and immune pathways that can work independently or in concert. 213 , 216 Alterations in the host’s diet, use of antibiotics, exposure to psychosocial stress, or irregularities in the immune system may shift the relative proportions of bacterial species, resulting in a disruption of the microbiota’s composition and functionality as dysbiosis. 214 Subsequently, the intestinal epithelial barrier is compromised. Harmful substances and microorganisms in the intestinal tract could enter the bloodstream, triggering an immune response that may lead to systemic inflammation. The onset of systemic inflammation may allow inflammatory mediators to cross over the BBB and impact microglia, further exacerbating neuroinflammation. 213 , 217 This process is accompanied by imbalanced neurotransmission, 218 which ultimately leads to neuronal degeneration and damage. Overall, the microbiota-gut-brain axis hypothesis establishes a connection between the peripheral immune system and the CNS, offering a fresh perspective for AD research. Moreover, drugs and biomarkers 219 related to the gut microbiome are potentially considered. However, the investigation of this mechanism is still in an early stage. The exact mechanisms by which the gut microbiome affects brain activity or its connections with other pathological features of AD remain unclear.
Abnormal autophagy
Autophagy, a highly conserved metabolic degradation process, maintains cellular homeostasis by delivering intracellular protein aggregates and damaged organelles to lysosomes for degradation and recycling. 220 , 221 It primarily occurs via three types: microautophagy, chaperone-mediated autophagy, and macroautophagy (commonly referred to as autophagy). 222 Microautophagy is the simplest pathway in which cytoplasmic substrates enter vesicles formed by morphological changes in lysosomal or endosomal membranes, and are ultimately degraded within the lysosome. 220 , 223 , 224 Chaperone-mediated autophagy involves chaperone proteins recognizing and binding to specific protein sequences (KFERQ-like motifs), facilitating substrate transfer to lysosomes through interactions with lysosomal membrane proteins (LAMP2A). 224 , 225 , 226 Macroautophagy, the main subtype, is primarily regulated by mTORC1 for activating the unc-51-like autophagy activating kinase 1 (ULK1) complex and dephosphorylating transcription factor EB (TFEB) to induce autophagy. Under the regulation of autophagy-related protein complexes, a phagophore forms and gradually expands to a sealed autophagosome. The autophagosomes then move retrogradely along microtubules to the microtubule organizing center, which is rich in lysosomes. They fuse with lysosomes to form autolysosomes, where substrate degradation occurs. In certain instances, autophagosomes could first merge with endosomes to form amphisomes, which then fuse with lysosomes. 222 , 224 , 227 , 228 , 229 However, the abundant accumulation of autophagic vacuoles in swollen (malnourished) neurons is observed to have a linkage with Aβ/APP-βCTF, suggesting that autophagy clearance is severely disrupted under pathological conditions and is closely linked to amyloid pathology. 115 , 225 , 230 This makes autophagy a focal point in recent AD pathogenesis research. There is increasing evidence indicating that genetic factors, reduced expression of related proteins, and defective vesicular transportation are potential causes of autophagy pathway disruptions. These disruptions interfere with clearance mechanisms involving substrate engulfment, autophagosome formation, autophagosome-lysosome fusion, and lysosomal structure and function. 227 , 229 In AD, autophagy defects mediate the disruption of protein homeostasis networks (production and extracellular secretion of Aβ, abnormal aggregation of tau protein) and lead to the accumulation of damaged organelles, such as dysfunctional mitochondria. 231 In summary, abnormalities of autophagy are intimately related to the onset and progression of AD. There is a growing emphasis on the involvement of chaperone-mediated autophagy, 232 contributions of glial cell autophagy, 233 , 234 and the precise causes of mitochondrial autophagy disorders. 235 Autophagy-stimulating drugs including small molecule therapies and gene therapies, have shown significant neuroprotective potential in various AD animal models, suggesting a potential intervention option. 220 , 222 , 231 , 236 , 237 However, the challenges posed by the broad targets of autophagy modulators, and lack of appropriate in vivo autophagic flux detection methods, hinder further clinical applications of these drugs. 222 , 227
Signaling pathways linked to AD pathogenesis
Neuroinflammatory signaling.
Several pathological factors in AD, such as Aβ, pro-inflammatory cytokines, and oxidative stress, activate microglia and initiate downstream signaling pathways such as MAPK, NF-κB, and PI3K/Akt. The activation of these pathways further promotes the activation of microglia and the production of inflammatory mediators, exacerbating neurotoxicity. 238 , 239 , 240 ERK, JNK, and p38 MAPK are three primary MAPK signaling pathways that may activate transcription factors such as AP-1 and NF-κB to release pro-inflammatory cytokines like TNF-α, IL-1β, and NO. 241 , 242 NF-κB can be co-regulated by multiple pathways including MAPK and PI3K/Akt to enhance transcriptional activity, thus promoting the expression of pro-inflammatory and pro-oxidant enzyme genes. 239 , 243 , 244 A recently identified microRNA, miR-25802, found to be overexpressed in AD, likely plays a crucial role in exacerbating disease pathology. This microRNA may regulate the polarization of microglial cells towards a pro-inflammatory phenotype through the modulation of the KLF4/NF-κB signaling pathway. Such alterations can further aggravate key pathological features in the 5xFAD mouse model including increased deposition of Aβ plaques and deficits in learning and memory. 245 The NF-κB signaling pathway significantly impacts the expression of components related to the NLRP3 inflammasome, such as NLRP3 protein, ASC, pro-IL-1β, and pro-IL-18. The NLRP3 inflammasome activates caspase-1 through its assembly and activation processes. Activated caspase-1 can cleave gasdermin D (GSDMD), triggering pyroptosis and releasing IL-1β, IL-18, and ASC specks into the extracellular environment. This may exacerbate the spread of inflammation and neuronal death. 246 , 247 , 248 , 249 Additionally, the connection between NF-κB signaling and NLRP3 inflammasome activation with AD tau pathology has garnered significant attention. Inactivated NF-κB pathways in microglia may reduce the seeding and amplification of tau proteins in microglia, thus rescuing cognitive deficits in young PS19 mouse models, yet the accumulation of tau inclusions in neurons of aged PS19 mice warrants further investigation. 250 According to recent studies, pro-inflammatory cytokines like IL-1β may induce an increase in tau transcription in human primary neurons by activating the NF-κB signaling pathway in neurons. Brain-derived tau proteins may activate the inflammatory response in microglia via the TLR2/MyD88/NF-κB pathway. 251 Research by Ising et al. suggests that tau proteins can activate the NLRP3 inflammasome, which then promotes excessive tau phosphorylation and aggregation by affecting specific tau kinases and phosphatases. 252 These findings reveal the complex interplay between inflammatory responses and tau pathology, providing a more comprehensive understanding of AD’s molecular mechanisms. The activation of the cGAS-STING signaling pathway in AD also plays a crucial role in neuroinflammation. Studies by Xie et al. found that the abnormal accumulation of double-stranded DNA in the cytoplasm may bind to the cytoplasmic DNA sensor (cGAS), thereby specifically triggering the STING-interferon (IFN) signaling pathway in microglia, promoting the expression and secretion of inflammatory cytokines. The relationships between microglia and other cells, such as astrocytes and neurons, further extend the scope of inflammation, forming a complex network of inflammatory regulation. 253 , 254 It is noteworthy that persistent neuroinflammation may lead to the infiltration of peripheral immune cells (such as T cells, B cells, monocytes, and neutrophils), yet the mechanisms of this infiltration and impacts on AD’s disease progression remain to be studied. 254 , 255 , 256 A recent study using a special 3D human neuroimmune axis model explored the interactions between infiltrative peripheral immune cells and innate immune cells in AD. The study found that C-X-C motif chemokine ligand 10 (CXCL10) and its receptor CXCR3 play key roles in regulating the infiltration of CD8+ T cells into the brain, and the infiltrated CD8+ T cells appear to interact with microglia to jointly promote AD’s neurodegeneration. 257 In the APP-PS1 transgenic mouse model, Unger et al. found that CD8+ T cells might affect brain activity by regulating genes associated with neuronal and synaptic functions, providing new clues about the potential mechanisms of CD8+ T cells in AD neuronal dysfunction and cognitive deficits. 258 Additionally, TREM2 has emerged as a potential therapeutic target due to its potential role in early AD in modulating neuroinflammation, Aβ plaque deposition, and cognitive abilities. 259 Recent research findings continue to reveal the potential mechanisms by which TREM2 plays a neuroprotective role in AD. For instance, Wang et al. suggest that the anti-inflammatory mechanisms induced by TREM2 may be associated with the PI3K-Akt-FoxO3a axis. The PI3K/Akt pathway, upregulated by TREM2, may regulate the activity and subcellular localization of FoxO3a, thereby reducing the expression levels of pro-inflammatory cytokines. 259 Moreover, TREM2 has been reported to bind with high affinity to C1q (the initiator of the classical complement pathway) to effectively inhibit the classical complement pathway, protecting synapses from abnormal phagocytosis and loss in AD. 260
Lysosomal dysfunction
Lysosomes rely on a rich array of acidic hydrolases to selectively degrade and recycle both intracellular and extracellular materials, playing a crucial role in maintaining cellular homeostasis. 261 Lysosomal dysfunction is considered a critical factor in the development of many diseases, 261 which may manifest as impaired acidification, abnormal expression of lysosomal enzymes, lysosomal membrane stability issues, transport defects, and defects in autophagosome/endosome-lysosome fusion. These issues may disrupt lysosomal degradation pathways, including the autophagy-lysosomal pathway and endosomal-lysosomal system, leading to the accumulation of pathological proteins and damaged organelles, further disrupting the cellular environment. 261 , 262 , 263 A key factor affecting lysosomal function is the pH controlled by the vacuolar (H+)-ATPase (V-ATPase), which uses the energy from ATP hydrolysis to drive H + from the cytoplasm into the lysosome. Other factors such as Cl - , Ca 2+ , and Na + ion channels/transporters also interact with the luminal pH and collectively regulate the lysosomal acidic environment. 264 , 265 In AD, lysosomal acidification deficits may weaken the clearance of Aβ, ultimately leading to the accumulation of extracellular Aβ plaques. 115 This phenomenon indicates that lysosomal-related clearance system dysfunction might be one of the early events in the progression of AD and has become a focus of current AD research. It has been reported that the PS1 holoprotein may facilitate N-glycosylation of the V0a1 subunit of V-ATPase and its trafficking from the endoplasmic reticulum (ER) to lysosomes, thereby promoting the assembly and maturation of V-ATPase. 266 However, there are inconsistent views on a series of events caused by defects in PS1, including impaired maturation of V0a1 in lysosomes, V-ATPase dysfunction, and lysosomal acidification defects. 267 , 268 Calcium dysregulation associated with PS1 has been proposed as a potential cause of endolysosomal defects. 268 Lee et al. once again affirmed the link between lysosomal acidification dysfunction and V-ATPase, further elucidating that aberrant lysosomal acidification mediates transient receptor potential cation channel mucolipin subfamily member 1 (TRPML1) overactivation, resulting in dysregulation of lysosomal calcium ions. Moreover, they demonstrated that solely reversing lysosomal calcium ion levels in cellular models failed to impact lysosomal acidity and autophagic function beneficially. 269 Another study suggested that PS1 mutations may lead to the opening of another calcium ion channel, two pore segment channel 2 (TPCN2), whose markedly enhanced activity greatly promotes lysosomal calcium efflux and lysosomal alkalinization. 270 Thus, the relationship among PS1 gene mutations or deficiencies, lysosomal acidification, and lysosomal calcium ion dysregulation warrants further investigation. Recent research has also revealed the impact of other AD-related genes on lysosomal dysfunction. For instance, increased phosphorylation of APP β-C-terminal fragment (βCTF) Tyr682 inhibited the assembly and activity of V-ATPase by binding to the V0a1 subunit, resulting in elevated lysosomal pH and impaired degradation capacity. 271
Cholesterol metabolism
Cholesterol is abundant in the brain, serving as a critical component of the myelin sheath and the membranes of neural cells, including neurons and glial cells. 272 The balance between cholesterol synthesis, transport, metabolism, and clearance is crucial for neuronal growth, synaptic plasticity, and learning and memory functions. 273 , 274 , 275 In AD, cholesterol biosynthesis and catabolism are impaired, contributing to the progression of AD through mediation of Aβ, tau, inflammation, and other pathological changes. 275 , 276 The connection between cholesterol and Aβ may be related to lipid rafts, which are cholesterol-rich microdomains on the plasma membrane. These rafts may facilitate the colocalization of APP with its cleaving enzymes, enhance the activities of β and γ secretases, and influence the endocytosis of APP, thereby mediating its amyloidogenic pathway. 276 , 277 With the assistance of cholesterol transporter APOE, astrocyte-derived cholesterol could be transferred to neuronal membranes, regulating cholesterol-dependent lipid clusters (also known as lipid rafts) on neurons to promote Aβ generation. Differences in cholesterol levels caused by different APOE isoforms may be related to their cellular expression and regulatory mechanisms. 278 Additionally, different APOE isoforms have varying impacts on Aβ pathology. Compared to APOE3 and APOE2, APOE4-mediated pathways of Aβ clearance are impaired, and APOE4 exhibits a higher affinity interaction with Aβ, potentially driving a more severe Aβ plaque burden, 119 , 121 , 123 making it one of the strongest genetic risk factors for AD. Cholinergic dysregulation associated with ApoE4 also contributes to tau pathology. For instance, in chimeric human cerebral organoids (chCOs), astrocytes and neurons carrying the APOE4 genotype could jointly promote tau phosphorylation in neurons, closely linked to the role of APOE4 in increasing cholesterol levels and lipid droplet content, suggesting that APOE4 may affect tau phosphorylation in AD by influencing lipid metabolism. 279 Litvinchuk et al. revealed a potential synergistic effect between APOE4 and tau pathology, wherein APOE4 may induce the abnormal accumulation of certain cholesterol esters in glial cells. This accumulation subsequently triggers the activation of glial cells, the release of inflammatory cytokines, infiltration of T-cells, and synaptic damage. 280 Furthermore, activation of the inflammation-related NLRP3 inflammasome signaling pathway in different types of neural cells was closely associated with high cholesterol load, which triggered neuroprotective properties in activated microglia but promoted oxidative stress in neurons, further enhancing the expression of NLRP3 inflammasomes, inducing neuronal pyroptosis, and impairing the phagocytic capacity of microglia. 281
Mitochondrial dysfunction
Mitochondria are the primary source of cellular energy and mediate a multitude of biological processes including biosynthesis, redox balance, calcium signaling, and apoptosis, serving as the core drivers of vital activities. 282 , 283 Observations in AD-afflicted brains of regionally reduced glucose metabolism and alterations in several mitochondrial enzyme activities suggest mitochondrial dysfunction. 284 This is primarily manifested by defects in energy metabolism, increased oxidative stress, calcium ion imbalance, and abnormal mitochondrial dynamics, all potentially leading to neuronal dysfunction and even apoptosis, exacerbating the neurodegenerative changes in AD. 282 , 285 Moreover, AD pathological biomarkers could directly impact mitochondrial function, creating a vicious cycle. Aβ inhibits the activity of key mitochondrial enzymes such as electron transport chain enzyme complex IV, pyruvate dehydrogenase (PDH), and α-ketoglutarate dehydrogenase (αKGDH), reducing the efficiency of electron transfer, diminishing ATP synthesis, and stimulating the production of ROS. 286 Additionally, Aβ interacts specifically with mitochondrial Aβ-binding alcohol dehydrogenase (ABAD), impeding the binding of NAD to ABAD and inducing ROS production. 287 , 288 The generation of ROS and the imbalance of the antioxidant system further damage mitochondrial DNA, lipids, and proteins, aggravating mitochondrial dysfunction and cellular apoptosis. 283 , 289 As the most common secondary messenger in cells, the importance of calcium ions is self-evident, and their homeostatic disruption is a significant factor in mitochondrial damage. 290 Aβ may increase cytosolic calcium levels and impair mitochondrial calcium buffering functions through various pathways including plasma membrane receptors and calcium channels, 291 enhanced ER calcium release, 292 and the mitochondrial inner membrane calcium channel MCU. 293 , 294 This leads to mitochondrial calcium overload, causing cyclophilin D (CypD) to relocate from the mitochondrial matrix to the inner membrane, promoting the formation of the mitochondrial permeability transition pore (mPTP), further inhibiting ATP synthesis, activating oxidative stress, and apoptosis. 289 , 295 Moreover, tau is also associated with mitochondrial calcium imbalance, and due to the critical role of tau in microtubule structure and function, its abnormal phosphorylation and aggregation may adversely affect mitochondrial axonal transport, impacting local metabolic needs and overall neuronal function. 296 , 297 Impairments in mitochondrial fission and fusion mechanisms, as well as mitophagy, are also areas of concern in AD. Alterations in the expression levels of proteins related to fission/fusion processes (such as Opa1, Drp1, MFN1/2, Fis1) 298 and post-translational modifications of Drp1 299 , 300 may bias mitochondria towards excessive fission, increasing mitochondrial fragmentation, leading to damage in mitochondrial energy biology and accumulation of mitochondrial DNA damage. 283 , 301 Fragmented mitochondria significantly obstruct mitophagy in AD, where PINK1/parkin-regulated mitophagy is a focal point of current research. 302 , 303 , 304 PINK1 accumulates on the outer membrane of damaged mitochondria and activates parkin, which then ubiquitinates several mitochondrial outer membrane proteins to initiate the autophagic pathway, engulfing damaged mitochondria to maintain mitochondrial health and function. 305 PINK1/parkin cascades related to Aβ, APP-CTFs, tau, and the APOE4 isoform could lead to the accumulation of damaged mitochondria. 306 The accumulation of Aβ and increased p-tau, synaptic dysfunction, in turn, negatively regulate mitophagic activity, accelerating the pathological progression of AD. 304
Calcium signaling
Intracellular calcium could originate from the opening of plasma membrane calcium channels, such as voltage-gated and ligand-gated calcium channels, and the release of organelles like the ER and mitochondria. 307 , 308 , 309 Calcium plays a multifaceted role in regulating gene expression, neurotransmitter release, membrane excitability, and inducing synaptic plasticity. 310 , 311 Additionally, plasma membrane calcium ATPases (PMCA), the sarco/ER calcium ATPase (SERCA), the sodium-calcium exchangers (NCX), and Ca 2+ -binding proteins also regulate cytosolic calcium concentration. 312 , 313 , 314 , 315 Maintaining this calcium homeostasis is fundamental to calcium signaling, and disruption in cytosolic calcium concentration gradients, as well as abnormalities in calcium signaling pathways, may lead to neurodegenerative diseases such as AD and Parkinson’s disease (PD), cardiovascular diseases, and metabolic disorders. 315 , 316 , 317 , 318 In AD, enhanced activity of L-type VGCCs, potentially related to their interaction with Aβ/tau, promotes excessive calcium influx into cells. 319 Studies have shown that using L-type calcium channel blockers could mitigate the upregulation of L-type VGCCs and abnormal calcium influx induced by Aβ. 320 Ligand-gated calcium channels such as NMDAR and α7nAChR, highly permeable to Ca 2+ , are closely associated with Aβ. 308 Overactivation of NMDARs by Aβ leads to abnormal calcium influx, triggering a cascade of downstream signaling events, resulting in dendritic spine loss, reduced distribution of NMDARs on neuronal membranes, impaired synaptic plasticity, and ultimately, cognitive decline. 321 , 322 Complexes formed by Aβ with α7-nAChR efficiently promote Aβ internalization and increased calcium influx, further affecting extracellular Aβ plaque accumulation and synaptic transmission. 308 Abnormal intracellular calcium signaling could also impact various organelles such as the ER, mitochondria, and lysosomes. The impaired function of SERCA and/or overactivation of calcium release channels (InsP3R and ryanodine (RyR) receptors) on the ER could facilitate the activation of the ER stress response. 307 The ER regulates the expression of unfolded protein response (UPR)-related target genes by increasing the formation of transcription factors ATF4, XBP1, and ATP6, providing cellular stress tolerance. However, persistently high-stress levels may trigger ER-mediated apoptosis. 323 Mitochondrial physiological functions are closely linked to calcium transfer between the ER and mitochondria, a process crucially mediated by MAMs. 324 , 325 , 326 Under the influence of Aβ, the expression of some MAM-related proteins, such as IP3Rs and VDAC1, is significantly increased, 325 , 327 , 328 leading to mitochondrial Ca 2+ overload, inhibition of normal ATP synthesis, and potential release of apoptotic signals. 329 Research has found that lysosomal acidity is also within the realm of calcium regulation, where excessive Ca 2+ released from the ER-resident RyR receptor can impair the function of lysosomal V-ATPase, causing lysosomal acidification defects, reducing lysosomal protease activity, and leading to the accumulation of p-tau. 330
Insulin signaling
Insulin regulates glucose metabolism, neuronal growth and survival, synaptic plasticity, and cognition, 331 , 332 , 333 functions closely linked to two main insulin signaling pathways: phosphatidylinositol 3-kinase (PI3K)-Akt and Ras/Raf-MAPK. 334 , 335 The PI3K-Akt pathway is a crucial component of insulin signaling, and in AD brains, there is observed a decrease in IRS-associated PI3K activity and reduced phosphorylation of Akt kinase. 336 , 337 Lower levels of Akt activation weaken the inhibition of glycogen synthase kinase-3 (GSK-3), which in turn positively affects the phosphorylation of tau protein and the production of Aβ. 333 , 338 , 339 mTORC1, a downstream molecule of Akt, also serves as a critical nexus linking insulin signaling with the autophagy system. Its role in the inhibitory phosphorylation of IRS1, synaptic protein synthesis, synaptic plasticity, and autophagy regulation is significantly correlated with the accumulation of pathological protein aggregates and impaired learning and memory functions in AD. Some drugs targeting mTORC1 have been demonstrated in animal studies to effectively inhibit abnormal mTORC1 activation, thereby enhancing autophagy, reducing Aβ and tau pathology, and helping to delay cognitive decline. However, some studies express divergent views on the activity of mTORC1 in AD. 340 Furthermore, the increased production of inflammatory mediators like TNF-α and the activation of stress kinases such as JNK, PKR, and IKK could promote the inhibitory serine phosphorylation of IRS-1, downregulate insulin signaling in the brain, and induce AD neurological dysfunction. 331 , 341
Dysregulated neurotrophic signaling pathway
Neurotrophic factors not only promote the survival, growth, and differentiation of neurons but are also crucial for maintaining synaptic plasticity and neuronal signaling functions. 342 , 343 In AD, key neurotrophic factors include NGF and brain-derived neurotrophic factor (BDNF), which exert their effects through specific receptors such as tropomyosin-related kinase (Trk) and p75 NTR . 15 In AD, there is a reduction in the conversion of proNGF to mature NGF and an enhancement in the degradation of mature NGF, 344 leading to a deficiency in mature NGF and accumulation of proNGF in the brain. The lack of mature NGF may promote the phosphorylation of APP at T668, reducing its binding to TrkA and affecting its subcellular localization, thus increasing amyloidogenic processing of APP and Aβ production. 345 The accumulation of proNGF and downregulation of TrkA (pro-survival signal) levels favor the predominance of pro-apoptotic signaling mediated by p75 NTR , further promoting the degeneration of basal forebrain cholinergic neurons. 346 , 347 Downregulation of BDNF expression leads to weakened BDNF signaling in AD. 348 This weakened signaling triggers the activation of JAK2/STAT3 and C/EBPβ signaling pathways in the AD brain and inhibits downstream Akt signaling molecules, 349 thereby promoting the activation of asparagine endopeptidase (AEP; also called δ-secretase) to cleave APP and tau proteins. 350 , 351 The cleaved tau fragments could bind to TrkB receptors, further inducing neuronal apoptosis. 349 A study suggested that impaired BDNF nutritional signaling also stimulated the expression of APP and PS1 to exacerbate amyloidogenesis. 352 Similarly, Aβ can interfere with common neuroprotective signaling pathways, such as the Raf-MAPK/ERK pathway and the PI3K-Akt pathway, initiated by the binding of BDNF to TRKB, inducing cortical neurons into a dysfunctional state. 353 According to recent research, microglial repopulation/self-renewal contributed to the restoration of BDNF expression and activation of the BDNF/TrkB neurotrophic signaling pathway, significantly reversing cognitive deficits in 5xFAD mice. This suggests that BDNF may provide potential benefits for AD treatment through its positive modulation of impaired synaptic plasticity and cognitive memory. 354
BBB dysfunction
The BBB is formed by components such as endothelial cells, astrocytes, and pericytes, along with the basement membrane, and together with other cells like microglia and neurons, they constitute the neurovascular unit (NVU). 355 , 356 The BBB not only allows highly selective permeability of substances entering and exiting through specialized structures (seal off adjacent BECs) but also dynamically regulates cerebral blood flow through the process of neurovascular coupling, maintaining homeostasis and neuronal function in the CNS. 355 , 357 , 358 , 359 Dysfunction of the BBB includes disruption of BBB integrity (or BBB leakage), changes in BBB transport functions, reduced cerebral blood flow, and neuroinflammation. Some evidence suggests that in AD, dysregulation of tight junction proteins, increased matrix metalloproteinase signaling, and degeneration and loss of pericytes may all contribute to BBB leakage, leading to the accumulation of numerous blood-derived neurotoxic proteins in the brain, causing neuroinflammation and oxidative stress. 356 , 360 , 361 , 362 Disruption of the BBB may also lead to ischemic/hypoxic brain damage and increase Aβ production. 358 Abnormal expression of transport proteins/receptors in the BBB, such as downregulation of LRP1 which exports Aβ from the brain to the blood, impaired function of Pgp, and upregulation of RAGE that facilitates the entry of Aβ from the blood into the brain, could be potential reasons for impaired Aβ clearance and substantial accumulation in the brain. 363 Reduced activity and expression of the GLUT-1 transporter in the BBB suggest decreased glucose uptake and utilization by the brain, 360 , 363 which may further exacerbate cerebrovascular degeneration, BBB breakdown, and Aβ pathology in models overexpressing APP, inducing neurodegeneration and cognitive deficits (Fig. 4 ). 364

Signaling pathways linked to AD pathogenesis. a Neuroinflammatory signaling. It involves interactions among various cell types, which influence neuroinflammation by activating multiple pathways. This leads to the production of inflammatory mediators and neuronal damage, accelerating the pathological progression of AD. b Lysosomal dysfunction. It may be related to impairments in V-ATPase-mediated lysosomal acidification and/or dysregulation of lysosomal calcium homeostasis. However, the specific mechanisms require further investigation to be definitively determined. c Aberrant cholesterol metabolism. d Mitochondrial dysfunction. Mitochondria in AD are damaged in various ways, including impairments in oxidative phosphorylation, calcium homeostasis, mtDNA, mitochondrial fusion and fission, axonal transport, and mitophagy. These dysfunctions lead to impaired energy production and increased oxidative stress. 283 e Calcium signaling in AD. Under physiological conditions, calcium ions follow a strict concentration gradient. In AD, the elevated cytosolic calcium concentration and calcium-responsive signaling cascades adversely affect protein folding in the ER, energy production in mitochondria, and lysosomal acidity. 307 g Insulin signaling in AD. f Dysregulated neurotrophic signaling pathway. h BBB dysfunction. The disruption of the integrity and alterations in the transport functions of BBB lead to the abnormal entry and exit of certain substances into and out of brain tissue, resulting in neuronal damage and further exacerbating the pathological progression of AD 644
Clinical trials of AD
Biomarkers for ad diagnosis.
The National Institute on Aging and Alzheimer’s Association (NIA-AA) proposed a research framework to define the biology of AD using Aβ deposition, pathologic tau, and neurodegeneration AT(N) biomarkers. 365 The current established biomarkers mainly include imaging biomarkers, cerebrospinal fluid (CSF) biomarkers, and blood biomarkers. Molecular imaging techniques like magnetic resonance imaging (MRI) and positron emission tomography (PET) are commonly used to detect structural and functional brain activity in vivo. 366 Specifically, structural MRI (sMRI) assesses hippocampal and entorhinal cortex atrophy in the medial temporal lobe, 18 fluorodeoxyglucose ( 18 FDG)-PET detects reduced glucose metabolism in the posterior cingulate and temporoparietal lobes, and PET imaging shows Aβ and tau deposition. 366 , 367 , 368 However, sMRI and ( 18 FDG)-PET indicate neurodegeneration or neuronal injury in the AT(N) framework with limitations in specifically diagnosing AD. They cannot accurately differentiate AD from other neurodegenerative diseases with similar pathologies, such as frontotemporal degeneration and TDP-43 proteinopathies with medial temporal lobe atrophy. Additionally, the atypical AD and cerebrovascular diseases may also complicate the diagnosis. 2 , 369 , 370 , 371 Therefore, these methods typically need to be combined with other clinical information and assessment tools for a comprehensive evaluation of AD pathology. Amyloid PET and tau PET not only reflect the overall accumulation and spatial distribution of amyloid plaques and NFTs but may also detect abnormal brain changes earlier than neurodegeneration, thus providing opportunities for early intervention in the disease. 366 , 371 Studies have reported that amyloid PET exhibits 90% sensitivity and specificity in diagnosing AD, and tau PET can specifically identify AD dementia from other neurodegenerative diseases, showing higher diagnostic accuracy than MRI markers. 368
NIA-AA’s AT(N) research framework includes CSF biomarkers such as Aβ 42 (or the Aβ 42 / Aβ 40 ratio), phosphorylated tau (P-tau), and total tau (T-tau). Notably, P-tau181 concentration is the most accurate indicator for differentiating AD from non-AD dementia. 372 , 373 While amyloid and tau PET and CSF biomarkers specifically indicate AD-related pathology, they are not entirely equivalent. Studies show a highly negative correlation between amyloid PET and CSF results, whereas CSF P-tau and tau PET findings are inconsistent. This discrepancy is related to their respective representations of PHFs formation and pathological tau deposition, with the latter’s higher correlation to cognitive abilities supporting tau PET as the most effective method for predicting cognitive decline in AD. 365 , 374 A recent study indicated that within 20 years, abnormalities in CSF Aβ 42 , the ratio of CSF Aβ 42 to Aβ 40 , CSF P-tau181, CSF T-tau, CSF neurofilament light chain (NfL), and hippocampal volume (as detected by sMRI) appear in sequence before the clinical diagnosis of SAD. 375 This suggests that CSF biomarkers may reveal changes in the disease process earlier than imaging biomarkers. 7 Therefore, selecting effective and reliable biomarkers, considering their sensitivity and specificity, as well as the potential inconsistencies among different biomarkers, is crucial for determining the nature and pathological stage of the disease in clinical practice. Recently, more CSF biomarkers reflecting other biological processes in AD have emerged, such as axonal injury and synaptic dysfunction (NfL, neurogranin (NG), synaptosomal-associated protein 25, visinin-like protein 1), 366 , 367 , 372 neuroinflammation (TREM2, YKL40, S100B, glial fibrillary acidic protein (GFAP)), 371 , 376 , 377 , 378 changes in neurotrophic protein levels (BDNF and NGF), 379 BBB disruption (soluble platelet-derived growth factor receptor-β), 380 and metabolic changes (sphingomyelin, ceramide, fatty acid-binding protein 3, ubiquitin C-terminal hydrolase L1). 381 , 382 Extracellular vesicles (EV), crucial in AD pathology spread, have gained attention. Proteomic studies found elevated C1q levels in MCI and AD groups, and increased CatB concentration in CSF Aβ 42 -positive cases. These factors are potentially involved in early AD pathology through synaptic aberrant pruning and rapid abnormal metabolism of APP, respectively. They present potential CSF EV-related biomarkers pending further validation. 383 , 384 Blood biomarkers offer an economical, convenient, minimally invasive, and highly accessible diagnostic alternative. 385 , 386 , 387 Many CSF biomarkers (like Aβ, P-tau, NfL, GFAP) also show promising applications in blood, with advancements in highly sensitive analytical platforms and detection techniques enhancing diagnostic precision and reliability. 368 , 388 , 389 For instance, an innovative integrated proteomic assay accurately measured levels of 21 AD-related blood biomarkers, which jointly evaluated AD from five dimensions: neurodegeneration, inflammation, innate immunity, vascular function, and metabolic activity. Machine learning models built on this dataset have accurately classified AD/MCI and Aβ pathology across different ethnicities, demonstrating potential benefits in early disease screening, pathology progression monitoring, and assessing the clinical efficacy of treatments. 390 In summary, the emergence of AT(N) and non-AT(N) biomarkers has significantly improved the accuracy of AD diagnosis. The use of “composite biomarker panel” 390 (effective combination of biomarkers) could comprehensively reflect the biological state of AD and enhance diagnostic accuracy. This is of great importance for differentiating MCI/AD patients from cognitively normal individuals, distinguishing AD from other neurodegenerative diseases, and even identifying AD subtypes. However, AD-related comorbidities may reduce the diagnostic value of biomarkers. 391 , 392 , 393 For example, coexisting αSyn pathology in AD correlates with lower CSF P-tau181 and NG levels, 394 while comorbidity like hypertension lowers plasma Aβ concentration but increases plasma P-tau181 and P-tau217 levels. 388 , 395 Future research should focus on developing more AD-specific biomarkers while also identifying biomarkers for non-AD-related diseases, aiding in a clearer understanding of AD pathology and accurately distinguishing AD from other neurodegenerative diseases. 368
Clinical drugs
Traditional AD drugs (Fig. 5 ) are categorized into two classes: AChEIs (tacrine ( 3 ), donepezil ( 4 ), rivastigmine ( 5 ), galantamine ( 6 )) and NMDA receptor antagonists (memantine ( 7 )). 396 AChEIs boost postsynaptic stimulation by increasing both the level and the action duration of ACh, thereby enhancing cognitive and behavioral functions in patients. 397 Tacrine ( 3 ) was approved for AD treatment in 1993 and pulled from the market in 2013 due to its liver toxicity. Nevertheless, it has potential in the study of multitarget-directed ligands. 30 , 398 , 399 Second-generation AChEIs, including donepezil ( 4 ), rivastigmine ( 5 ), galantamine ( 6 ), are more selective. They exhibited fewer side effects or improved pharmacokinetic profiles, establishing them as first-line drugs for AD. 98 , 400 Although these drugs have been widely used, ongoing research focuses on optimizing dose, dosage form, routes of administration, and combination therapies to minimize adverse effects and improve patient compliance as much as possible. 401 , 402 , 403 The donepezil ( 4 ) transdermal patch, named Adlarity, was FDA-approved in 2022 for treating mild, moderate, and severe dementia of the Alzheimer type. 404 Its weekly dosing frequency showed bioequivalence to daily oral administration at the same dosage while presenting fewer gastrointestinal adverse events than oral administration. This also offers greater convenience compared to the once-daily rivastigmine ( 5 ) patch. 405 The application of nanocarriers is also being explored to deliver these cholinesterase inhibitors through intranasal administration, intravenous injection, and other methods. Nanocarriers play a crucial role in increasing drug concentrations, slowing drug release, and achieving excellent bioavailability. 401 , 406 , 407 Furthermore, the combination use of appropriate cholinesterase inhibitors, such as donepezil ( 4 ) and galantamine ( 6 ), or the combination of cholinesterase inhibitors with other neurologic drugs, metal chelators, or antioxidants, may yield surprising effects in the management of cholinergic drugs in AD, including efficacy, tolerability, and safety. 402 , 408 Memantine ( 7 ) is an FDA-approved NMDA receptor antagonist for the treatment of moderate to severe stages of AD. It modulates glutamate transmission and dopamine receptors, exhibiting certain efficacy in improving patients’ cognitive function, daily living abilities, and behavior. 409 , 410 Namzaric ( 8 , fixed-dose combination memantine ( 7 ) extended-release/donepezil ( 4 )) also provides another treatment option for patients with moderate to severe AD. 51 These drugs primarily function by modulating neurotransmitter levels but cannot alter the course of the disease, 409 , 411 which are instructive for designing new drugs. In 2017, a review 412 proposed “disease modifying therapy for AD”, which aims to intervene in the fundamental biological mechanisms to halt the disease’s progression and provide enduring therapeutic benefits to patients. Sodium oligomannate ( 9 , GV-971), an oligosaccharide extracted from marine algae, was conditionally approved in China in 2019 amidst ongoing debates regarding its mechanism of action and therapeutic efficacy. 54 , 413 Sodium oligomannate ( 9 , GV-971) was postulated to counteract AD by inhibiting neuroinflammation triggered by gut dysbiosis and disrupting the formation of Aβ fibrils. 56 , 414 Further research indicated that sodium oligomannate ( 9 , GV-971) altered the composition and abundance of the gut microbiome in a sex-dependent manner in both APPPS1-21 and 5xFAD models. This modulation influenced microbial metabolism and peripheral inflammation, regulated the activation state and functionality of microglia, and thereby reduced neuroinflammation and amyloidosis. 415 Currently, two phase IV clinical trials (NCT05181475 and NCT05058040) are ongoing to further investigate its efficacy and safety, with an expected continuation until 2025. Aducanumab ( 1 ), lecanemab ( 2 ), and donanemab ( 10 ) are monoclonal antibodies targeting Aβ, each of which has met with differing outcomes: Aducanumab ( 1 ) 416 , 417 received controversial FDA accelerated approval in 2021; Lecanemab ( 2 ) 61 gained traditional approval in 2023; Donanemab ( 10 ) 63 has completed phase III trials and is in the process of market authorization. Their status is closely linked to their mechanisms. Aducanumab ( 1 ) binds to 3-7 amino acids of Aβ, targeting soluble oligomers and insoluble fibrils. 418 , 419 Lecanemab ( 2 ), associated with the E22G Aβ, 420 showed stronger binding to soluble Aβ aggregates (oligomers and protofibrils) than aducanumab ( 1 ). 421 Donanemab ( 10 ) targets pyroglutamate-modified Aβ, binding specifically to plaques. 419 All three have shown efficacy in clearing Aβ plaque and slowing cognitive decline, but the risks of amyloid-related imaging abnormalities (ARIA) and their treatment costs are noteworthy. 422 , 423 , 424 Brexpiprazole ( 11 ), commonly prescribed for depression and schizophrenia, targets serotonin, dopamine, and norepinephrine receptors. It is known to help mitigate agitation in individuals with AD. 425 , 426 , 427 These innovative medicines delve deeper into AD mechanisms and present diverse target choices, holding the potential to halt or reverse AD progression. Further studies are needed to understand drug mechanisms, assess long-term efficacy, and ensure safety. In addition, the unfavorable risk-benefit ratio in AD makes drug repurposing a common approach. The long, high-cost, and resource-heavy process of developing AD medications, coupled with their high rate of failure, has led to growing interest in repurposing medications originally designed for other conditions, including cancer, cardiovascular diseases, psychiatric disorders, diabetes, and other neurological diseases. 428 , 429 These drugs are noted for their extensive safety and tolerance profiles, as well as their potential for multiple uses. 428 , 430 Additionally, the advancement of artificial intelligence (AI)-based computational tools is facilitating drug repurposing, presenting a promising strategy AD drug development. 431 , 432 , 433

Approved drugs for AD by FDA/China. Notably, the definition of disease-modifying therapies, capable of producing enduring and impactful changes in the clinical progression of AD, was first proposed in 2017. 412 (The numbers 1, 2 ,…… 8, 9 in the figure represent the drug identifiers defined by the authors)
As documented on ClinicalTrials.gov, the AD research landscape encompasses 187 clinical trials, spanning phase I, II, and III, specifically targeting AD and MCI attributed to AD. Among these trials, 36 drugs are in phase III, 87 in phase II, and 31 in phase I. 434 The major mechanisms of action center around: 1) neurotransmitter receptors, including AChE, NMDA receptor, 5-hydroxytryptamine receptor, nicotinic α7 receptor, and adrenoceptor; 2) Aβ, including the reduction of Aβ production (such as γ-secretase inhibitors and modulators, BACE1 inhibitors, and α-secretase activators), prevention of Aβ aggregation, and enhancing Aβ clearance (vaccines and antibodies); 3) tau proteins (phosphorylation modulators, aggregation inhibitors, microtubule stabilizers, antibodies, and vaccines); and 4) inflammation (NSAIDs, microglia modulators). 434 , 435 , 436 , 437 The majority of phase II and III trials center around neurotransmitter receptors and Aβ mechanisms, while tau and inflammation drugs are more prominent in phase II, often featuring repurposed compounds. Typical/Representative AD drugs in advanced clinical stages are detailed in Table 1 . Semagacestat ( 12 , LY-450139) was the first γ-secretase inhibitor to enter phase III clinical trials. A clinical trial (NCT00594568) aimed at assessing the long-term progression of AD found deterioration in cognitive and functional status across all trial groups. Additionally, participants experienced adverse reactions such as gastrointestinal symptoms, skin cancer, and infections, which are speculated to be related to the inhibition of other γ-secretase substrates, including notch, CD44, ErbB4, and cadherin. 438 , 439 , 440 , 441 Avagacestat ( 13 , BMS-708163) is an orally administered γ-secretase inhibitor that exhibited greater selectivity for APP-C99 compared to semagacestat ( 12 , LY450139). 440 Phase I studies indicated its effectiveness in reducing Aβ levels. However, during a phase II study assessing its safety and tolerability in patients with prodromal AD (NCT00890890), adverse events including gastrointestinal issues and skin cancer were observed in the high-dose treatment group. 442 Researchers have explored inhibiting β-secretase (BACE1) as an alternative to γ-secretase inhibitors due to its higher selectivity for APP, aiming to reduce Aβ production. 443 Umibecestat ( 14 , CNP520), a fourth-generation BACE1 inhibitor, initially showed good safety and tolerability in early clinical studies. 444 , 445 However, two phase II/III trials (NCT02565511 and NCT03131453), conducted on older individuals with high risk of AD (carriers of the APOE4 allele) but without cognitive impairment, were terminated prematurely. This decision was made due to observations of mild cognitive decline and brain atrophy in participants. 446 , 447 Elenbecestat ( 15 , E2609), a fourth-generation BACE1 inhibitors, was among the last BACE1 inhibitors to reach phase III clinical trials. 448 A phase III trial (NCT02956486) assessing effectiveness and safety in early-stage AD patients was terminated due to an unfavorable risk-benefit ratio. More specifically, literature 446 , 449 indicates that the termination was due to the lack of help in cognition and the emergence of side effects such as nightmares, weight loss, rash, and liver damage. ALZ-801 ( 16 ), an orally administered small molecule drug with tramiprosate as its active ingredient, exhibited effective anti-Aβ oligomer activity without binding to plaques, potentially reducing the risk of ARIA associated with plaque clearance. 450 , 451 In interim results from its phase II trial (NCT04693520), the drug lowered biomarker levels and showed the potential to slow the decline in memory and learning abilities in early AD patients carrying the APOE4 gene (either APOE4/4 or APOE3/4). 425 The ongoing phase III clinical trial (NCT04770220) aims to further validate these positive results regarding efficacy and safety in APOE4 homozygous individuals with early AD, with the study expected to continue until 2024. Varoglutamstat ( 17 , formerly PQ912), the first small molecule glutaminyl cyclase inhibitor to enter phase II clinical trials, targets an enzyme that catalyzes the conversion of glutamate to pyroglutamate at the N-terminus of Aβ. This modification results in Aβ forms that are more prone to form toxic aggregates. 452 , 453 In its phase IIa study (NCT03919162), varoglutamstat ( 17 , formerly PQ912) demonstrated acceptable safety and tolerability, as well as a reduction in working memory decline. 454 The ongoing phase IIb VIVIAD trial (NCT04498650) aims to further explore its long-term safety, tolerability, effects on cognition, and impact on AD biomarkers. 455 Solanezumab ( 18 , LY2062430) is an antibody targeting the intermediate domain of Aβ, effective against soluble, monomeric, non-fibrillar forms of Aβ, thus promoting the dissolution of plaques. 456 In the initial two phase III trials (NCT00905372 and NCT00904683) evaluating the drug’s efficacy compared to a placebo in patients with mild to moderate AD, the drug did not significantly delay cognitive or functional decline. However, it appeared to potentially alter the disease course in patients with mild AD. In the expedition3 trial (NCT01900665), aimed at further validating the drug’s efficacy in patients with mild AD, the drug was declared unsuccessful in 2016, as it failed to meet its primary endpoints. 457 , 458 , 459 Gantenerumab ( 19 ) is a subcutaneously administered antibody capable of binding to two regions of Aβ – the N-terminal and the central structural domain. 460 It targets soluble oligomers, protofibrils, and plaques. 461 Two phase III trials (NCT03444870 and NCT03443973) were recently terminated. In these trials, when assessing the efficacy and safety of gantenerumab ( 19 ) in participants with early (prodromal to mild) AD, the drug showed little clinical benefit in slowing cognitive decline, potentially due to limited clearance of amyloid plaques, with 5.0% participants experienced amyloid-related imaging abnormalities-effusion (ARIA-E) related side effects. 461 , 462 Tideglusib ( 20 ), a non-ATP-competitive GSK-3β inhibitor, exhibits neuroprotective and anti-inflammatory properties. 463 In its phase II study (NCT01350362), which evaluated the drug’s efficacy, safety, and tolerability in patients with mild to moderate AD, it did not meet some primary and secondary endpoints. 464 TRx0237 ( 21 , LMTX) is a tau aggregation inhibitor. 465 All phase III trials have now been completed or terminated. Two earlier studies (NCT01689233 and NCT01689246) conducted on participants with mild AD and mild to moderate AD, respectively, indicated that the drug demonstrated good safety and potential benefits as a monotherapy. 466 , 467 Another phase III trial (NCT03446001) aimed to further confirm the safety and efficacy of 16 mg/day monotherapy compared to placebo in participants with mild to moderate AD, with results pending disclosure. 468 Bepranemab ( 22 , UCB0107), an antibody targeting the central region of tau, potentially inhibits tau aggregation and propagation. 469 A phase II study (NCT04867616) for AD is undergoing to evaluate its efficacy, safety, and tolerability in patients with MCI or mild AD. E2814 ( 23 ) is a monoclonal antibody that targets the tau microtubule-binding region, thereby inhibiting tau protein aggregation and seed propagation. 470 The drug is currently undergoing three clinical trials. A phase I/II trial (NCT04971733) aims to assess the safety, tolerability, and target engagement of E2814 ( 23 ) in participants with dominantly inherited AD (DIAD), with completion expected in 2025. The other two phase II/III trials (NCT01760005 and NCT05269394) aim to evaluate the efficacy of the combination of E2814 ( 23 ) and lecanemab ( 2 ) in early-onset AD. These trials respectively use the changes in cognitive measures and tau PET as their primary outcome measures and are expected to conclude in 2027. AADvac1 ( 24 ) is the first tau vaccine to enter clinical trials, 469 aiming to inhibit tau aggregation, remove tau aggregates, prevent pathological spread, and slow disease progression. A phase II study (NCT02579252) evaluating the drug’s safety and efficacy in patients with mild AD showed that AADvac1 ( 24 ) was well-tolerated with no significant adverse reactions. However, its clinical efficacy requires further validation. 471 NE3107 ( 25 , formerly HE3286) is a small insulin sensitizer that inhibits inflammation. 425 A phase III clinical trial (NCT04669028) has been completed, aimed at testing the safety and efficacy of the drug in elderly patients with mild to moderate AD. The results indicated that the drug was well-tolerated and effectively slowed down the rate of cognitive decline in participants, significantly improving cognitive function. 472 ALZT-OP1 ( 26 ) is a combination treatment of cromolyn sodium and ibuprofen. It induces the transformation of microglial cells into a pro-phagocytic/neuroprotective activation state and blocks Aβ aggregation. 473 ALZT-OP1 ( 26 ) has completed a phase III study (NCT02547818) assessing its safety and efficacy in subjects with evidence of early AD. The study aimed to determine whether the combination therapy of ALZT-OP1 ( 26 ) could slow down or reverse cognitive and functional decline in early-stage AD participants. AL002 ( 27 ) is a TREM2-specific monoclonal antibody that activates TREM2 to enhance microglial function, thereby reducing Aβ plaque formation and attenuating neurite dystrophy. 474 A phase II study (NCT04592874) is currently underway to evaluate the efficacy and safety of AL002 ( 27 ) in participants with early-stage AD. Masitinib ( 28 ) is a potent and selective tyrosine kinase inhibitor targeting multiple aspects of AD, including inhibition of microglia and mast cell activation, modulation of Aβ and tau protein signaling pathways, and prevention of synaptic damage. 475 It is currently undergoing a phase III clinical trial (NCT05564169). The objective of this study is to confirm the efficacy of masitinib ( 28 ) as an adjunct therapy to cholinesterase inhibitors and/or memantine ( 7 ) in improving cognitive and functional abilities in patients with mild to moderate AD. 476 Repurposed drugs include nilvadipine ( 29 ), a calcium channel blocker for the treatment of hypertension, and pioglitazone ( 30 ), a drug initially developed for diabetes. Nilvadipine ( 29 ) displays various properties, such as decreasing Aβ production, increasing cerebral blood flow, and exerting anti-tau and anti-inflammatory activities. A phase III trial (NCT02017340) testing the efficacy and safety of nilvadipine ( 29 ) in participants with mild to moderate AD indicated that, while the drug demonstrated good safety, it did not show significant benefits in slowing cognitive decline in AD patients. 477 Pioglitazone ( 30 ) is a PPARγ agonist widely used in the treatment of T2D. 478 Two phase III clinical trials (NCT01931566 and NCT02284906) assessed the safety and efficacy of the drug in participants with AD-induced MCI but were terminated due to insufficient efficacy.
In summary, the development of AD drugs has faced numerous challenges. Factors contributing to the suboptimal performance of drugs include the selection of drug targets, the use of biomarkers and animal models in experimental designs, and other issues such as late treatment initiation, dose-dependent side effects, challenges in BBB permeability, and the heterogeneous presentation of patients. 182 , 479 , 480 In the extensively researched Aβ hypothesis, Aβ stands as the most direct drug target. However, the structural polymorphism of Aβ, including monomers, soluble oligomers, protofibrils, and amyloid plaques, along with numerous pathogenic variants, complicates the selection of precise targets and adds to the complexity of designing effective drugs. 481 When Aβ antibodies, such as bapineuzumab ( 31 ), did not yield significant therapeutic effects, research shifted towards inhibiting the formation of Aβ. 109 , 170 However, the side effects associated with targeting β- and γ-secretases arise because these enzymes have a wide range of substrates that are vital in other physiological processes. 170 In addition, the overemphasis on the Aβ hypothesis has also hindered the emergence of diverse new targets. 482 , 483 Biomarkers play a crucial role in patient selection, biological effect detection, dose optimization, and monitoring response progress, with recent approvals of Aβ monoclonal antibodies benefiting from new and accurate biomarkers. 83 , 423 The disparity in drug performance between preclinical and human trials has driven the evolution of animal models. Current AD animal models have shifted from single genetic mutation models to multi-gene transgenic models, and consider non-genetic pathogenic factors and species differences to more accurately simulate the AD progression process. 484 , 485 , 486 , 487 While immunotherapy appears to be the most advanced therapeutic strategy, primarily targeting traditional targets such as Aβ and tau, a noticeable paradigm shift is occurring toward small-molecule therapeutic modalities. 435 These modalities, characterized by their simplicity, maturity, and adaptability, provide a promising avenue for emerging targets. The development of a new generation of small-molecule drugs for AD is thus an exciting prospect. Furthermore, diverse mechanisms of inhibition, including selective, dual-targeted, allosteric, covalent, PROTACs, and PPI-targeted approaches, are enhancing drug-like properties, safety, and efficacy. This multifaceted approach aims to expedite the development of valuable drugs for both traditional and emerging targets, streamlining the drug development cycle and mitigating associated challenges.
Potential therapeutic drugs
The multifactorial nature of AD onset, coupled with the complex interactions among these factors, poses significant challenges to drug development. The limited efficacy of traditional medications, combined with the high failure rates in clinical drug development due to insufficient efficacy or adverse effects, has raised the bar for the development of the next generation of AD drugs. These drugs aim to furnish a repertoire of diverse and precise treatments tailored to individual patients and their distinct pathological processes. Progress in understanding the pathophysiological mechanisms, combined with advancements in drug development technologies, has paved the way for the discovery of novel drugs. Details of next-generation compounds in AD are outlined in Table 2 .
Selective inhibitors
Given the association of pan-inhibitors with cytotoxicity and adverse events, coupled with a deepening understanding of the physiological functions of pathological proteins, the development of selective inhibitors has advanced significantly. 488 , 489 , 490 These inhibitors are capable of specifically targeting categories, subtypes, and structural domains, 491 potentially providing more pronounced benefits in terms of efficacy, safety, and tolerability. 67 Kadsuranin [(+)-2] ( 32 ) and gomisin N [( − )-2] ( 33 ), which are two stereoisomers of schisandrin B extracted from the fruits of S. chinensis , have been shown to effectively inhibit GSK-3β in an ATP-competitive manner. Administering these compounds has been shown to effectively mitigate memory deficits and markedly reduce the expression of phosphorylated tau in the hippocampus in the APP/PS1 double-transgenic mice. 492 Targeting less conserved substrate binding sites, as opposed to ATP binding sites, might offer advantages in terms of drug specificity, functional regulation, and safety. 493 , 494 For example, compound 34 demonstrated these benefits. 495 As the role of GSK-3α in promoting Aβ production and tau phosphorylation in AD models is recognized, selective inhibition of GSK-3α has emerged as a promising therapeutic strategy. 494 , 496 , 497 The GSK-3α ATP-competitive inhibitor 35 could cross the BBB and significantly reduce tau phosphorylation at pThr231 in neonatal rat brains, potentially delaying early pathological progression in AD. 497 It is noteworthy that simultaneous inhibition of both GSK-3α and GSK-3β could excessively activate the wnt/β-catenin pathway, leading to abnormal cell proliferation and other detrimental effects. 496 , 498 Therefore, the ideal state for selective drugs is to ensure efficacy while providing a suitable therapeutic window for safety. For instance, the selective GSK3β inhibitor OCM-51 ( 36 ) could achieve a beneficial balance between reducing tau phosphorylation and preventing overactivation of the β-catenin signaling pathway at appropriate doses. 499 Additionally, leveraging the dynamic changes of targets may be a potential strategy for developing selective inhibitors. Given that overexpression of dual-specificity tyrosine phosphorylation-regulated kinase 1 A (DYRK1A) may influence the initial progression of AD through mechanisms including the hyperphosphorylation of pathologically relevant substrates such as tau, APP, PS1, regulation of axonal transport of APP, and participation in the selective splicing of tau pre-mRNA, 500 , 501 , 502 the compound dp-FINDY ( 37 ) effectively targets the spatial dynamic changes in the ATP-binding site between the DYRK1A folding intermediate and the folded state, specifically acting on the folding intermediate. 503 This may reduce excessive interference with numerous physiological substrates of this target and offer a novel perspective in selective drug design. Histone deacetylases (HDACs) are epigenetic regulators that modulate gene expression by removing acetyl groups from lysine residues on proteins, affecting processes like cell proliferation, differentiation, and development. 504 , 505 Among them, HDAC6 has two catalytic domains and a C-terminal zinc finger domain, interacts with tau and α-tubulin, and is involved in the degradation of protein aggregates, mitochondrial transport, and cognitive memory, 506 , 507 , 508 , 509 making it relevant to AD pathology. HDAC6 inhibitors typically consist of three parts: a zinc-binding group (ZBG), a cap group, and a hydrocarbon motif connecting the cap and ZBG. 510 , 511 Their selectivity often involves strong hydrophobic interactions between the cap group and a large surface area on HDAC6, known as the “L1 loop pocket”. 507 , 512 Compound 38 , incorporating cap group of melatonin and ferulic acid, enhanced HDAC6 selectivity while providing significant antioxidant capacity, alleviating spatial working and non-spatial long-term memory deficits in Aβ 25-35 -injected mice at lower doses. 513 Compound 39 achieved strong HDAC6 selectivity through interaction with another specific pocket on HDAC6, inhibiting tau hyperphosphorylation and aggregation. It demonstrated neuroprotective activity through ubiquitination mechanisms and improved learning and memory in animal models, presenting a potential therapeutic avenue for AD. 514 In most cases of selective inhibitor development, research initially relies on the scaffold of lead compounds to provide basic affinity and molecular framework. Subsequent modifications enhance drug-target binding, solubility, metabolic stability, and BBB permeability. Compounds 40 and 41 were identified through a combination of docking-based virtual screening and pharmacophore modeling from an in-house oncology compound library. Their shared scaffold may offer new insights for casein kinase 1δ (CK1δ) inhibitor development. 515 In AD, c-Jun N-terminal kinase3 (JNK3) activation is closely associated with neuronal damage, amyloid deposition, and the formation of tau tangles. 516 Hah et al. have conducted in-depth studies on this target, continuously refining and developing several generations of compounds based on the structure of pan-JNK inhibitor 42 , which was identified through an in-house kinase-focused library screening. These compounds yielded significant improvements in potency, selectivity, and pharmacokinetic properties while maintaining key interactions with JNK3. 517 , 518 , 519 Recently studied compounds 43 and 44 exhibited excellent performance in three behavioral tests of homozygous APPswe/PS1dE9 double transgenic mouse models and 3xTg mouse dementia models (Fig. 6a ). 519

a Chemical structures of selective inhibitors 32 - 44 . b Dual-target inhibitors 45 - 50 . c GSK-3 degrader 62 , as well as PhosTACs 63 and 64 . (The numbers 32, 33 ,…… 51, 62, 63, 64 in the figure represent the compound identifiers defined by the authors)
The development of selective inhibitors benefits the understanding of the roles played by different targets and their subtypes in AD, and it may also reduce the risk of side effects. Some adverse effects may originate from the off-target proteins. Differences in amino acids, explicit water molecules, spatial conformation and dynamics between the target and other proteins binding sites could serve as the basis for drug selectivity. However, in AD drug development, designing inhibitors with high selectivity poses significant challenges when faced with highly conserved or homologous binding pockets. The discovery of additional pockets on the target enzyme, target optimization (identifying substitutable targets), and the use of computational tools may offer new strategies. Nevertheless, the complexity and diversity of AD mechanisms suggest the difficulties of targeting specific targets and their limited impact on the disease progression. In addition to targeting specific enzymes, drugs aim to improve efficacy and reduce adverse reactions by focusing on specific distribution and functions in the pathological stage. For instance, PROTAC technology leverages E3 ligases, which may be selectively expressed in certain tissues, to drive the targeted degradation of specific targets, 520 offering significant opportunities for AD treatment. Covalent drugs also exhibit impressive performance in selective targeting, 521 potentially providing novel inhibitory approaches for kinases such as CK1, which have previously only been targeted with non-covalent ATP competitive inhibitors. 522 Further drug development techniques will also be discussed below, aiming to enhance drug efficacy and safety within a broader scope of selectivity.
Dual-target inhibitors
Given the multifactorial nature of AD 523 and the suboptimal effects of single-target drugs, 524 the search for effective dual- or multi-target inhibitors has emerged as a new research trend. These inhibitors act on one or more targets with additive or synergistic effects, aiming to increase efficacy, prolong therapeutic effects, minimize side effects, and lower drug doses. 68 , 69 , 525 Compared with combined therapies, they further reduce the risk of drug-drug interactions and simplify administration, making treatment safer, more effective, and more convenient for patients. 524 , 525 From a biochemical standpoint, growing evidence supports a link between cholinergic abnormalities and other pathophysiological features of AD, including abnormal Aβ and tau. Consequently, cholinesterase inhibitors have become a fundamental approach in AD treatment. 526 Targeting both AChE and Butyrylcholinesterase (BuChE) not only alleviates cognitive impairment in AD patients by increasing ACh levels but also serves as a disease-modifying agent, delaying the formation of Aβ plaques. 527 , 528 , 529 The dual inhibitor of AChE and BuChE, compound 45 , significantly enhanced the learning and memory abilities of aged AD mice. The significant alleviation in Aβ burden, anti-inflammatory and antioxidative effects, and enhanced synaptic transmission activity were also observed in the hippocampus. 530 Given the elevated activity of monoamine oxidase-B (MAO-B) observed in AD, dual inhibition of AChE and MAO-B holds promise for synergistic effects on cholinergic system recovery and Aβ plaque formation, along with potential benefits in alleviating oxidative stress injury. 531 Ladostigil ( 46 ), an AChE/MAO-B inhibitor developed through a pharmacophore fusion strategy, 532 has completed a clinical phase II trial (NCT01429623). The trial aimed to evaluate the safety and efficacy of low-dose ladostigil ( 46 ) in patients with MCI. The results indicated that the drug was well-tolerated and safe, seemingly possessing the potential to delay the progression of AD. 533 Compound F681-0222 ( 47 ) leveraged the functional interplay between BACE1 and AChE to decrease soluble Aβ 42 levels in the brain tissue of APPswe/PS1dE9 transgenic mice. 534 The simultaneous modulation of AChE and GSK-3β has the potential on improving cholinergic and tau protein signaling pathways. 523 , 535 AChE/GSK-3β inhibitors 48 536 and 49, 537 developed through a pharmacophore linkage strategy, exhibited promising results by significantly inhibiting tau hyperphosphorylation and ameliorating cognitive disorders in scopolamine-treated ICR mice. Additionally, inhibiting AD-related phosphodiesterases (PDEs) could consequently enhance synaptic transmission and mitigating cognitive deficiencies. 538 , 539 Compound 50 is a dual-inhibitor of AChE and PDE4D. It exhibited exceptional neuroprotection against cell death and more substantial anti-neuroinflammatory effects in the hippocampus of AD model mice induced by Aβ 25-35 than the combined treatment of donepezil ( 4 ) and rolipram ( 51 ) (Fig. 6b ). 540
For diseases with complex etiologies, single-target drugs often struggle to interfere with the complete network regulation of the disease and tend to produce significant toxicity. The design and application of dual-targeted and multi-targeted inhibitors place a greater emphasis on the interrelations of pathological factors, enhancing the convenience of medication for patients. Multi-target drugs can act on multiple interconnected targets in AD. Although their activity on a single target may be lower compared to single-target drugs, the synergistic effects of multi-target modulation result in a total effect greater than the sum of the individual effects, leading to better efficacy and fewer adverse reactions. The primary strategies include pharmacophore-linked and pharmacophore-merged methods. 541 Although these approaches facilitate drug design on a technical level, relying on a limited set of known SARs for pharmacophores may somewhat limit the structural diversity of the drugs and narrow the range of targets. Inspiration for drug design often draws from natural products and computer-aided screening. Additionally, the physicochemical properties, pharmacokinetic characteristics, and toxicity of the drugs are critical factors that must be carefully considered during the design processes.
Allosteric modulators
Allosteric modulators typically attach to regions distinct from the orthosteric site of receptors, inducing conformational changes to regulate the affinity and/or efficacy of orthosteric ligands, or to directly modulate receptor activity with positive, negative, or neutral effects. 542 , 543 , 544 , 545 This precise tuning of receptor activity has revitalized the development of anti-γ-secretase drugs in the field of AD. Allosteric modulators of γ-secretase encourage the production of shorter, less toxic Aβ subtypes, and even potentially minimize effects on Notch and some other substrates. Some γ-secretase modulators (GSMs) also exhibited promising safety outcomes in preclinical studies and clinical trials. 546 , 547 , 548 Compared to orthosteric sites, allosteric sites often have lower conservation and greater diversity, 549 providing new avenues for drug development targeting highly homologous subtypes, such as nAChR and mAChR. The α7 nAChR subtype presents a potential approach for treating AD due to its high expression in cognitive function-related brain areas and interaction with Aβ. 550 , 551 Selective positive allosteric modulators (PAMs) targeting the α7 nAChR subtype, such as compound 52 , slowed the decline of episodic/working memory in amnesia mouse models. Unlike orthosteric agonists, 52 did not cause receptor desensitization even with repeated dosing, and is currently being evaluated in clinical trials for its efficacy and safety in mild to moderate AD patients. 552 M1-mAChR positive allosteric modulators (M1-PAMs), such as BQCA ( 53 ) and PF06764427 ( 54 ), achieve subtype selectivity through allosteric effects but have significant agonistic activity that may lead to side effects like diarrhea. 544 , 553 The respective optimized derivatives of BQCA ( 53 ) and PF06764427 ( 54 ), compounds 55 . 554 and 56, 555 require further in vitro and in vivo studies to evaluate their pharmacokinetic properties and allosteric modulation effects. Moreover, achieving signaling bias through allosteric modulation could enhance the safety of M1-mAChR drugs, making it a key consideration in the development of M1-mAChR allosteric ligands. 542 , 544 , 545 Beyond the cholinergic system, allosteric drugs find broad application in AD. For example, chlorphenylalic acid PS48 ( 57 ) targets PDK-1 allosteric pocket to restore Akt insulin responsiveness. The drug reduced Aβ toxicity without over-regulating insulin signaling, presenting a promising strategy for AD prevention or treatment. 556 In a phase I study (NCT05077501), the novel Trk receptor PAM ACD856 ( 58 ). 557 demonstrated good safety and tolerability, as well as favorable pharmacokinetic properties, potentially benefiting neurotrophic factor signaling. 558 Several reviews 70 , 559 , 560 , 561 have extensively summarized allosteric modulation strategies targeting other proteins such as GSK-3β, NMDARs, AMPA receptors, and RIPK1 (Fig. 7a ).

a Chemical structures and modification schemes of allosteric modulators 52-57 . b covalent inhibitors 59 - 61 . c Compounds 65 - 74 target the PPI network. (The numbers 52, 53 ,…… 57, 59, 60, 61, 65 ,…… 74 in the figure represent the compound identifiers defined by the authors)
Allosteric modulation, with its distinctive features of low-conservation binding sites, subtype or even signaling pathway selectivity, saturated allosteric effects, 562 and subtle-tuning of target function, exhibits strong appeal in AD drug development. Nonetheless, the discovery and development of allosteric drugs are facing challenges. Advantages of molecular docking and dynamics simulations, X-ray crystallography, and cryo-electron microscopy have facilitated the discovery of allosteric sites to enhance our understanding of allosteric modulation. 563 , 564 However, the complexity of allosteric modulation requires a number of in vitro and in vivo studies to thoroughly assess and analyze the functional effects of compounds and the factors influencing their characteristics. 564 Clearly, the potential benefits for AD cognitive deficits and the safety of allosteric drugs still need broader experimental data to support further optimization. 544 , 546
Covalent inhibitors
Covalent inhibitors, which form covalent bonds with their target proteins, rely on the specificity and stability of these interactions to exhibit superior potency, selectivity, and duration of action. This mechanism offers patients a convenient therapeutic option. 521 , 565 Based on experiences in cancer treatment and other diseases, the development of AD covalent drugs also has a broad prospect. In cancer therapy, covalent inhibitors often target cysteine residues with acrylamide warheads. 565 , 566 , 567 Based on this, compound 59 , which features an acrylamide warhead, can covalently bind to cysteine in GSK-3β. It significantly reduced the expression of APP and p-tau in the hippocampus of AD mice and improved spatial learning and memory abilities. 464 A widely studied Ru(III) anticancer drug, KP1019 ( 60 ), reveals a unique anti-Aβ strategy. Unlike conventional methods that inhibit Aβ production and aggregation, KP1019 ( 60 ) counteracted Aβ toxicity to neuronal cell models by promoting the formation of soluble high-molecular-weight Aβ aggregates. 568 This suggests that metal-based covalent inhibitors have promising potential in AD drug development. The electrophilic warheads and targeting residues of covalent inhibitors are continuously being developed. For example, the 6H8 ( 61 ) fragment, obtained through NMR screening from the Maybridge library, may act as a covalent warhead targeting the pathological substrate APP of γ-secretase, thereby hindering Aβ production. 569 , 570 This could be a supplementary method to avoid potential side effects of γ-secretase inhibitors. 569 In summary, the application of covalent inhibitors to some undruggable targets (such as Aβ, tau, and APPTM) has broadened the possibilities of drug design. The characteristics of covalent inhibitors are expected to reduce the required dosage and frequency of administration, thereby improving patient compliance and offering a new strategy for AD treatment. However, the potential toxicity of covalent inhibitors has always been a concern. Improving the selectivity of covalent inhibitors is critical and can be optimized through various means, including adjusting the reactivity and reversibility of the electrophile (warhead), 571 , 572 non-covalent scaffolds, dosage, etc. Relevant literature has discussed these aspects (Fig. 7b ). 565 , 567 , 573
The ubiquitin-proteasome system (UPS) is one of the primary protein degradation pathways within the cell. However, in AD, the dysfunction of this clearance pathway becomes a significant contributor to the accumulation of pathological proteins. 574 The PROTACs exploit the UPS system to precisely target specific proteins, improving the accuracy and speed of protein degradation. 575 Various reviews 574 , 575 have consolidated information on PROTACs with potential applications in AD. These PROTACs target tau protein, phosphokinase GSK-3β, HDACs, BET proteins, and transthyretin (TTR)-Aβ interaction, exhibiting characteristics such as low dosage requirements, high efficacy, and high target selectivity. As technology continues to advance, PROTACs undergo continuous refinement. For example, the GSK-3 degrader PT-65 ( 62 ), developed through click chemistry, exhibited a more prolonged effect on p-tau than its GSK-3 warhead (a GSK-3 inhibitor). This may help reduce dosing frequency. 576 Additionally, phosTAC7 ( 63 ) 577 and tau2-8 ( 64 ) 578 ingeniously leverage the flexibility of PROTACs to create targeted dephosphorylation strategies. In summary, PROTACs represent a burgeoning technology in AD drug development, specifically targeting dysfunctional enzymes, misfolded proteins, and even PPI in AD through the rational utilization of the UPS clearance system. However, PROTACs are still facing challenges. Limitations include the restricted choices of E3 ligases, primarily CRBN and VHL, and the considerable molecular weight of compounds that cause poor BBB penetration. Notably, while PROTACs can alter the existing pathological phenotype of AD, they cannot reverse the damage that has already occurred, particularly in addressing the genetic mutations associated with FAD (Fig. 6c ). 574
Targeting the PPI network
Protein-protein interactions (PPIs) are fundamental in maintaining cellular functions, while aberrant interactions between proteins are implicated in the pathogenesis of numerous diseases. 75 , 579 For instance, AD is characterized by the misfolding and aggregation of Aβ and tau proteins, involving a variety of molecular mechanisms and complex networks of PPIs. 580 , 581 , 582 Thus, disrupting these interactions may block some critical signaling pathways and potentially mitigate the pathological process of AD. Although large and flat PPI interfaces may be more conducive to peptide and protein drug targeting, 75 , 583 , 584 small molecule inhibitors also play a role in some AD-related PPIs due to their unique advantages. For example, Aβ can interact with the leukocyte immunoglobulin-like receptor B2 (LilrB2) and negatively mediate synapses and memory. 585 Compounds ALI6 ( 65 ) 586 and 66 587 can effectively block this interaction, which reverses the changes in cofilin signaling downstream of LilrB2 and the inhibition of neurite outgrowth, thus protecting neuronal cells from Aβ toxicity. In contrast, the interaction between Aβ and transthyretin (TTR) is a favored PPI, because it reduces Aβ aggregation and toxicity. 588 Iododiflunisal ( 67 , IDIF), luteolin ( 68 ), and three marketed drugs sulindac ( 69 ), olsalazine ( 70 ), and flufenamic ( 71 ) are small-molecule chaperones for the TTR/Aβ interaction. They all significantly reduced the caspase-3 activation in SH-SY5Y cells, protecting cells from apoptosis/death. Moreover, their good BBB penetration ability warrants their application in TTR target validation and positions them as potential candidates for AD clinical trials. 589 Kelch-like ECH-associated protein 1 (Keap1)-nuclear factor erythroid 2-related factor 2 (Nrf2), critical for regulating anti-oxidative stress, represents a PPI targetable by covalent inhibitors. 590 Its orally available inhibitor NXPZ-2 ( 72 ) effectively ameliorated Aβ-induced cognitive dysfunction in mice by increasing the expression levels of Nrf2 and downstream antioxidant enzymes. 590 However, issues of low solubility and lack of validation in transgenic AD models with NXPZ-2 ( 72 ) are presented, which was properly addressed by its analog 73 . 591 Additionally, another Keap1-Nrf2 PPI inhibitor 74 , which combined conformational features significantly similar to the Keap1-Nrf2 ETGE complex, revealed the unique inhibition mechanism and provided an innovative strategy for the development of new Keap1-Nrf2 PPI inhibitors. 592 In summary, inhibition or activation of fundamental pathological interactions presents an alternative therapeutic avenue for AD. PPI modulators precisely target pathological pathways in a reversible and mildly regulatory manner, preserving the physiological functions of proteins and thereby reducing severe side effects associated with excessive inhibition, thus offering higher safety levels. In addition, recent advances in computational analysis and model building also support the identification of specific, high-affinity PPI drug hits. These approaches systematically locate underutilized or optimal local interaction regions, simulating the dynamic and transient nature of PPIs, thereby presenting unlimited possibilities for efficient PPI drug discovery (Fig. 7c ). 593
Conclusions and prospects
AD is a progressive neurodegenerative disease characterized by declining memory and cognitive dysfunction. Pathological features such as Aβ plaques and NFTs in patients have been well documented. However, the existing hypothesis fails to fully elucidate the precise impact of these alterations on the onset and development of AD or the complex interactions among various pathological events. The focus on inflammatory responses and the immune system has led to speculation that certain pathogens such as Porphyromonas gingivalis , herpes simplex virus 1 (HSV1), and SARS-CoV-2 may play a role in AD, and the antimicrobial activity of Aβ may also partially supports the mechanism. 214 Some animal studies suggested that Porphyromonas gingivalis could translocate to the brain, closely linked to the deposition of Aβ and tau and the occurrence of neuroinflammation. 594 , 595 While some epidemiological data and preclinical studies suggest the association between HSV1 and AD, more research is needed to further validate and understand the relationship. 596 , 597 , 598 Research of both HSV1-infected mice and AD mouse models has revealed the gene MAM domain containing 2 (MAMDC2) exhibits significant expression in microglia, which results in high levels of I-IFNs to enhance antiviral responses in HSV1-infected mice and neuroinflammation in the AD animal model. 599 HSV1 may also impact Aβ pathology through mechanisms, such as continuous production and aggregation of Aβ within infected neurons via the activation of caspase 3, 600 and altering γ-secretase activity. 601 Many COVID-19 patients diagnosed with some long or post-acute sequelae of COVID-19 such as brain atrophy and memory decline, greatly increasing the risk of AD. 602 , 603 AD patients are also more susceptible to COVID-19, with higher risks of hospitalization and mortality in the patients with dementia and COVID-19. 604 This suggests a correlation between the two diseases. From a genetic perspective, some genes such as APOE4 and oligoadenylate synthetase 1 (OAS1) play important roles in susceptibility to both COVID-19 and AD. APOE4 as a significant genetic risk factor for AD also interacts with angiotensin-converting enzyme 2 (ACE2) to hinder SARS-CoV-2 infection and influence inflammation levels. 605 Some variants in the interferon-responsive gene OAS1 may lower its expression and potentially increase the likelihood of AD and severe COVID-19, through excessive release of pro-inflammatory signals in myeloid cells such as microglia and macrophages, further leading to cell death. 606 SARS-CoV-2 affects key pathological changes, such as Aβ, tau, and neuroinflammation, promoting cognitive impairment. Interaction between the SARS-CoV-2 Spike S2 subunit and γ-secretase could regulate γ-secretase cleavage of APP and increase Aβ production. 607 SARS-CoV-2 may facilitate the intercellular spread of tau aggregates by forming extracellular vesicles modified with spike S protein. 608 Upon entry into the host cell, it may cause cytokine storms and immune dysregulation, disrupt the BBB, and reduce Aβ clearance, ultimately resulting in neuroinflammation and Aβ aggregation. 602 Additionally, the upregulation of shared pathogenic kinases in COVID-19 and AD, such as epidermal growth factor receptors, vascular growth factor receptors, Bruton tyrosine kinase, spleen tyrosine kinase, c-ABL, and JAK/STAT, suggests potential interactions between immunological and neurological mechanisms. 609
The current approaches to addressing AD focus on three main aspects: prevention, early diagnosis, and treatment. Managing modifiable risk factors provides a pathway for AD prevention, which may help reducing cognitive decline and the risk of AD. In early diagnosis, various biomarkers of CSF, blood, urine, 610 saliva, 611 and retina, 612 may contribute to comprehensively reflecting the AD pathological process, serving as potential auxiliary tools that are more convenient, cost-effective, or less invasive. Pharmacotherapy is broadly employed in AD treatment; however, the efficacy or safety of most investigational and clinical drugs is not ideal. Factors such as dose-dependent adverse reactions, the inability to penetrate the BBB and achieve effective therapeutic concentrations, and variations in patient sensitivity and metabolic capacity may all influence outcomes. Here, we elucidate the issue from the perspective of the AD nature and drug development technologies. Firstly, the nature of AD may affect the choice of medication. For instance, the deficiency or mutation in aldehyde dehydrogenase (ALDH2) may influence melatonin administration, which could potentially benefit AD patients experiencing cardiac dysfunction. A study 14 found that in APP/PS1 mutant mice, the decrease in ALDH2 activity could lead to a cascade of downstream events, including disruption of mitochondrial integrity, accumulation of mitochondrial DNA in the cytoplasm, downregulation of the cGAS-STING-TBK1 signaling pathway, and inhibition of autophagy and mitophagy, ultimately resulting in cardiac disorders. Moreover, the beneficial effects of melatonin on mouse hearts, which depend on the regulation of ALDH2 activity, could not be assessed due to mutations or deficiencies in ALDH2. Secondly, appropriate drug development strategies provide the possibility of safe and effective drugs. These technologies may balance the efficacy and risk through targeting selection (single target/multiple targets, structurally similar targets, undruggable targets, active/non-active sites on targets, protein/PPI), the mode of action on targets (clearance, inhibition, or activation), and the duration and intensity of drug targets. Additionally, the burgeoning development of AI may impact AD due to its advantages in handling complex biomedical big data sets. 613 AI is currently making preliminary explorations in various aspects of AD, from detection and diagnosis to understanding disease mechanisms, biomarker discovery, clinical trial design, drug discovery, and prognosis prediction. Overall, AI’s integration into various facets of AD research holds promise for advancing our understanding of the disease. 614 , 615 , 616 , 617 , 618
WHO. A blueprint for dementia research. Geneva: World Health Organization (2022).
Knopman, D. S. et al. Alzheimer disease. Nat. Rev. Dis. Prim. 7 , 33 (2021).
Article PubMed Google Scholar
Querfurth, H. W. & LaFerla, F. M. Alzheimer’s disease. N. Engl. J. Med. 362 , 329–344 (2010).
Article CAS PubMed Google Scholar
2023 Alzheimer’s disease facts and figures. Alzheimers Dement . 19 , 1598–1695 (2023).
Abdelnour, C. et al. Perspectives and challenges in patient stratification in Alzheimer’s disease. Alzheimers Res. Ther. 14 , 112 (2022).
Article PubMed PubMed Central Google Scholar
Cummings, J. New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener. 16 , 2 (2021).
Graff-Radford, J. et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20 , 222–234 (2021).
Falgàs, N., Walsh, C. M., Neylan, T. C. & Grinberg, L. T. Deepen into sleep and wake patterns across Alzheimer’s disease phenotypes. Alzheimers Dement. 17 , 1403–1406 (2021).
Atri, A. The Alzheimer’s disease clinical spectrum: diagnosis and management. Med. Clin. North Am. 103 , 263–293 (2019).
Maciejewska, K., Czarnecka, K. & Szymański, P. A review of the mechanisms underlying selected comorbidities in Alzheimer’s disease. Pharm. Rep. 73 , 1565–1581 (2021).
Article Google Scholar
Dubois, B. et al. Biomarkers in Alzheimer’s disease: role in early and differential diagnosis and recognition of atypical variants. Alzheimers Res. Ther. 15 , 175 (2023).
Katabathula, S., Davis, P. B. & Xu, R. Comorbidity-driven multi-modal subtype analysis in mild cognitive impairment of Alzheimer’s disease. Alzheimers Dement. 19 , 1428–1439 (2023).
Gong, X. et al. A red-emitting mitochondria targetable fluorescent probe for detecting viscosity in HeLa, zebrafish, and mice. Anal. Methods 16 , 293–300 (2024).
Wang, S. et al. ALDH2 contributes to melatonin-induced protection against APP/PS1 mutation-prompted cardiac anomalies through cGAS-STING-TBK1-mediated regulation of mitophagy. Signal Transduct. Target Ther. 5 , 119 (2020).
Article CAS PubMed PubMed Central Google Scholar
Salasova, A., Monti, G., Andersen, O. M. & Nykjaer, A. Finding memo: versatile interactions of the VPS10p-Domain receptors in Alzheimer’s disease. Mol. Neurodegener. 17 , 74 (2022).
Marde, V. S. et al. Alzheimer’s disease and sleep disorders: Insights into the possible disease connections and the potential therapeutic targets. Asian J. Psychiatr. 68 , 102961 (2022).
Fehsel, K. & Christl, J. Comorbidity of osteoporosis and Alzheimer’s disease: Is ‘AKT ‘-ing on cellular glucose uptake the missing link? Ageing Res. Rev. 76 , 101592 (2022).
Gunes, S. et al. Biomarkers for Alzheimer’s disease in the current state: a narrative review. Int J. Mol. Sci. 23 , 4962 (2022).
Song, T. et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 72 , 101503 (2021).
Lloret, A. et al. When does Alzheimer’s disease really start? The role of biomarkers. Int J. Mol. Sci. 20 , 5536 (2019).
Porsteinsson, A. P. et al. Diagnosis of early Alzheimer’s disease: Clinical practice in 2021. J. Prev. Alzheimers Dis. 8 , 371–386 (2021).
CAS PubMed Google Scholar
Nedelec, T. et al. Identifying health conditions associated with Alzheimer’s disease up to 15 years before diagnosis: an agnostic study of French and British health records. Lancet Digit. Health 4 , e169–e178 (2022).
Zhang, X. X. et al. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 8 , 313–321 (2021).
PubMed Google Scholar
Logroscino, G. Prevention of Alzheimer’s disease and dementia: the evidence is out there, but new high-quality studies and implementation are needed. J. Neurol. Neurosurg. Psychiatry 91 , 1140–1141 (2020).
Crous-Bou, M., Minguillón, C., Gramunt, N. & Molinuevo, J. L. Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res. Ther. 9 , 71 (2017).
Omura, J. D. et al. Modifiable Risk Factors for Alzheimer Disease and Related Dementias Among Adults Aged ≥45 Years - United States, 2019. MMWR Morb. Mortal. Wkly Rep. 71 , 680–685 (2022).
Zhang, D. F. & Li, M. Toward a Full Understanding of Causal and Modifiable Risk Factors for Alzheimer’s Disease by Integrative Phenome-wide Association Studies. Biol. Psychiatry 93 , 756–758 (2023).
Silva, M. V. F. et al. Alzheimer’s disease: risk factors and potentially protective measures. J. Biomed. Sci. 26 , 33 (2019).
Beata, B. K. et al. Alzheimer’s Disease-Biochemical and Psychological Background for Diagnosis and Treatment. Int J. Mol. Sci. 24 , 1059 (2023).
Thakral, S. et al. Alzheimer’s disease: Molecular aspects and treatment opportunities using herbal drugs. Ageing Res. Rev. 88 , 101960 (2023).
Stanciu, G. D. et al. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 10 , 40 (2019).
Hampel, H., Lista, S. & Khachaturian, Z. S. Development of biomarkers to chart all Alzheimer’s disease stages: the royal road to cutting the therapeutic Gordian Knot. Alzheimers Dement. 8 , 312–336 (2012).
Sutphen, C. L., Fagan, A. M. & Holtzman, D. M. Progress update: fluid and imaging biomarkers in Alzheimer’s disease. Biol. Psychiatry 75 , 520–526 (2014).
Lista, S. et al. CSF Aβ1-42 combined with neuroimaging biomarkers in the early detection, diagnosis and prediction of Alzheimer’s disease. Alzheimers Dement . 10 , 381–392 (2014).
Reiman, E. M. Alzheimer disease in 2016: Putting AD treatments and biomarkers to the test. Nat. Rev. Neurol. 13 , 74–76 (2017).
Davis, K. L. & Powchik, P. Tacrine. Lancet 345 , 625–630 (1995).
Qizilbash, N. et al. Cholinesterase inhibition for Alzheimer disease: a meta-analysis of the tacrine trials. Dementia Trialists’ Collaboration. JAMA 280 , 1777–1782 (1998).
Jarrott, B. Tacrine: In vivo veritas. Pharm. Res. 116 , 29–31 (2017).
Watkins, P. B. et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 271 , 992–998 (1994).
de Los Ríos, C. & Marco-Contelles, J. Tacrines for Alzheimer’s disease therapy. III. The PyridoTacrines. Eur. J. Med. Chem. 166 , 381–389 (2019).
Birks, J. S. & Harvey, R. J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 6 , CD001190 (2018).
Cui, X. et al. Donepezil, a drug for Alzheimer’s disease, promotes oligodendrocyte generation and remyelination. Acta Pharm. Sin. 40 , 1386–1393 (2019).
Article CAS Google Scholar
Brewster, J. T. 2nd, Dell’Acqua, S., Thach, D. Q. & Sessler, J. L. Classics in Chemical Neuroscience: Donepezil. ACS Chem. Neurosci. 10 , 155–167 (2019).
Feldman, H. H. & Lane, R. Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 78 , 1056–1063 (2007).
Rösler, M. et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 318 , 633–638 (1999).
Coyle, J. & Kershaw, P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: effects on the course of Alzheimer’s disease. Biol. Psychiatry 49 , 289–299 (2001).
Scott, L. J. & Goa, K. L. Galantamine: a review of its use in Alzheimer’s disease. Drugs 60 , 1095–1122 (2000).
Marco-Contelles, J. et al. Synthesis and pharmacology of galantamine. Chem. Rev. 106 , 116–133 (2006).
Robinson, D. M. & Keating, G. M. Memantine: a review of its use in Alzheimer’s disease. Drugs 66 , 1515–1534 (2006).
Reisberg, B. et al. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 348 , 1333–1341 (2003).
Greig, S. L. Memantine ER/Donepezil: A Review in Alzheimer’s Disease. CNS Drugs 29 , 963–970 (2015).
Deardorff, W. J. & Grossberg, G. T. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s disease. Drug Des. Dev. Ther. 10 , 3267–3279 (2016).
Benek, O., Korabecny, J. & Soukup, O. A Perspective on Multi-target Drugs for Alzheimer’s Disease. Trends Pharm. Sci. 41 , 434–445 (2020).
Syed, Y. Y. Sodium Oligomannate: First Approval. Drugs 80 , 441–444 (2020).
Wang, T. et al. A phase II randomized trial of sodium oligomannate in Alzheimer’s dementia. Alzheimers Res. Ther. 12 , 110 (2020).
Xiao, S. et al. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res. Ther. 13 , 62 (2021).
Cummings, J. & Salloway, S. Aducanumab: Appropriate use recommendations. Alzheimers Dement 18 , 531–533 (2022).
Dhillon, S. Aducanumab: First Approval. Drugs 81 , 1437–1443 (2021).
Behl, T. et al. “Aducanumab” making a comeback in Alzheimer’s disease: An old wine in a new bottle. Biomed. Pharmacother. 148 , 112746 (2022).
Larkin, H. D. Lecanemab Gains FDA Approval for Early Alzheimer Disease. JAMA 329 , 363 (2023).
Harris, E. Alzheimer Drug Lecanemab Gains Traditional FDA Approval. JAMA 330 , 495 (2023).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388 , 9–21 (2023).
Sims, J. R. et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 330 , 512–527 (2023).
Hippius, H. & Neundörfer, G. The discovery of Alzheimer’s disease. Dialogues Clin. Neurosci. 5 , 101–108 (2003).
Hajjo, R., Sabbah, D. A., Abusara, O. H. & Al Bawab, A. Q. A Review of the Recent Advances in Alzheimer’s Disease Research and the Utilization of Network Biology Approaches for Prioritizing Diagnostics and Therapeutics. Diagnostics 12 , 2975 (2022).
Burns, S. et al. Therapeutics of Alzheimer’s Disease: Recent Developments. Antioxidants 11 , 2402 (2022).
Chen, J. et al. Targeting Bromodomain-Selective Inhibitors of BET Proteins in Drug Discovery and Development. J. Med. Chem. 65 , 5184–5211 (2022).
Feng, L. et al. Dual-target inhibitors of bromodomain and extra-terminal proteins in cancer: A review from medicinal chemistry perspectives. Med. Res. Rev. 42 , 710–743 (2022).
Tan, L. et al. Development of Dual Inhibitors Targeting Epidermal Growth Factor Receptor in Cancer Therapy. J. Med. Chem. 65 , 5149–5183 (2022).
Silva, G. M. et al. Allosteric Modulators of Potential Targets Related to Alzheimer’s Disease: a Review. ChemMedChem 14 , 1467–1483 (2019).
Wu, P., Clausen, M. H. & Nielsen, T. E. Allosteric small-molecule kinase inhibitors. Pharm. Ther. 156 , 59–68 (2015).
Boike, L., Henning, N. J. & Nomura, D. K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 21 , 881–898 (2022).
He, M. et al. PROTACs: great opportunities for academia and industry (an update from 2020 to 2021). Signal Transduct. Target Ther. 7 , 181 (2022).
Scott, D. E., Bayly, A. R., Abell, C. & Skidmore, J. Small molecules, big targets: drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 15 , 533–550 (2016).
Blazer, L. L. & Neubig, R. R. Small molecule protein-protein interaction inhibitors as CNS therapeutic agents: current progress and future hurdles. Neuropsychopharmacology 34 , 126–141 (2009).
Liu, P. P., Xie, Y., Meng, X. Y. & Kang, J. S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target Ther. 4 , 29 (2019).
Fedele, E. Anti-Amyloid Therapies for Alzheimer’s Disease and the Amyloid Cascade Hypothesis. Int. J. Mol. Sci. 24 , 14499 (2023).
Kepp, K. P. et al. The amyloid cascade hypothesis: an updated critical review. Brain 146 , 3969–3990 (2023).
Rubin, L. et al. Genetic Risk Factors for Alzheimer’s Disease in Racial/Ethnic Minority Populations in the U.S.: A Scoping Review. Front. Public Health 9 , 784958 (2021).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51 , 414–430 (2019).
Andrews, S. J. et al. The complex genetic architecture of Alzheimer’s disease: novel insights and future directions. EBioMedicine 90 , 104511 (2023).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54 , 412–436 (2022).
Hampel, H. et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 26 , 5481–5503 (2021).
Eid, A., Mhatre, I. & Richardson, J. R. Gene-environment interactions in Alzheimer’s disease: A potential path to precision medicine. Pharm. Ther. 199 , 173–187 (2019).
Boyd, R. J., Avramopoulos, D., Jantzie, L. L. & McCallion, A. S. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. J. Neuroinflamm. 19 , 223 (2022).
Rahman, M. A. et al. Emerging risk of environmental factors: insight mechanisms of Alzheimer’s diseases. Environ. Sci. Pollut. Res. Int. 27 , 44659–44672 (2020).
Galton, C. J., Patterson, K., Xuereb, J. H. & Hodges, J. R. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123 , 484–498 (2000).
Sirkis, D. W. et al. Dissecting the clinical heterogeneity of early-onset Alzheimer’s disease. Mol. Psychiatry 27 , 2674–2688 (2022).
Lam, B. et al. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res. Ther. 5 , 1 (2013).
Aisen, P. S. et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther. 9 , 60 (2017).
Morató, X. et al. Symptomatic and Disease-Modifying Therapy Pipeline for Alzheimer’s Disease: Towards a Personalized Polypharmacology Patient-Centered Approach. Int. J. Mol. Sci. 23 , 9305 (2022).
Selkoe, D. J. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 81 , 741–766 (2001).
Whitehouse, P. J. et al. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215 , 1237–1239 (1982).
Perry, E. Acetylcholine and Alzheimer’s disease. Br. J. Psychiatry 152 , 737–740 (1988).
Tagliavini, F. & Pilleri, G. Neuronal counts in basal nucleus of Meynert in Alzheimer disease and in simple senile dementia. Lancet 1 , 469–470 (1983).
Li, J., Sun, M., Cui, X. & Li, C. Protective Effects of Flavonoids against Alzheimer’s Disease: Pathological Hypothesis, Potential Targets, and Structure-Activity Relationship. Int J. Mol. Sci. 23 , 10020 (2022).
Auld, D. S., Kornecook, T. J., Bastianetto, S. & Quirion, R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol. 68 , 209–245 (2002).
Chen, Z. R., Huang, J. B., Yang, S. L. & Hong, F. F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 27 , 1816 (2022).
Chen, X. Q. & Mobley, W. C. Exploring the Pathogenesis of Alzheimer Disease in Basal Forebrain Cholinergic Neurons: Converging Insights From Alternative Hypotheses. Front. Neurosci. 13 , 446 (2019).
Hampel, H. et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141 , 1917–1933 (2018).
Giacobini, E., Cuello, A. C. & Fisher, A. Reimagining cholinergic therapy for Alzheimer’s disease. Brain 145 , 2250–2275 (2022).
Breijyeh, Z. & Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 25 , 5789 (2020).
Ferreira-Vieira, T. H., Guimaraes, I. M., Silva, F. R. & Ribeiro, F. M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 14 , 101–115 (2016).
Bekdash, R. A. The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer’s Disease. Int. J. Mol. Sci. 22 , 1273 (2021).
Berry, A. S. & Harrison, T. M. New perspectives on the basal forebrain cholinergic system in Alzheimer’s disease. Neurosci. Biobehav. Rev. 150 , 105192 (2023).
Majdi, A. et al. Amyloid-β, tau, and the cholinergic system in Alzheimer’s disease: seeking direction in a tangle of clues. Rev. Neurosci. 31 , 391–413 (2020).
Malik, R. et al. Overview of therapeutic targets in management of dementia. Biomed. Pharmacother. 152 , 113168 (2022).
Moreira, F. T. C., Sale, M. G. F. & Di Lorenzo, M. Towards timely Alzheimer diagnosis: A self-powered amperometric biosensor for the neurotransmitter acetylcholine. Biosens. Bioelectron. 87 , 607–614 (2017).
Schneider, L. S. et al. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J. Intern. Med. 275 , 251–283 (2014).
Morris, J. C. et al. Autosomal dominant and sporadic late onset Alzheimer’s disease share a common in vivo pathophysiology. Brain 145 , 3594–3607 (2022).
O’Brien, R. J. & Wong, P. C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 34 , 185–204 (2011).
Chen, G. F. et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharm. Sin. 38 , 1205–1235 (2017).
Lanoiselée, H. M. et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 14 , e1002270 (2017).
Pimplikar, S. W. et al. Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J. Neurosci. 30 , 14946–14954 (2010).
Lee, J. H. et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat. Neurosci. 25 , 688–701 (2022).
Sun, L., Zhou, R., Yang, G. & Shi, Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc. Natl Acad. Sci. USA 114 , E476–E485 (2017).
Ullah, R., Park, T. J., Huang, X. & Kim, M. O. Abnormal amyloid beta metabolism in systemic abnormalities and Alzheimer’s pathology: Insights and therapeutic approaches from periphery. Ageing Res. Rev. 71 , 101451 (2021).
Ayton, S. & Bush, A. I. beta-amyloid: The known unknowns. Ageing Res. Rev. 65 , 101212 (2021).
Raulin, A. C. et al. ApoE in Alzheimer’s disease: pathophysiology and therapeutic strategies. Mol. Neurodegener. 17 , 72 (2022).
Verghese, P. B., Castellano, J. M. & Holtzman, D. M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 10 , 241–252 (2011).
Koutsodendris, N., Nelson, M. R., Rao, A. & Huang, Y. Apolipoprotein E and Alzheimer’s Disease: Findings, Hypotheses, and Potential Mechanisms. Annu. Rev. Pathol. 17 , 73–99 (2022).
Bu, G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 10 , 333–344 (2009).
Serrano-Pozo, A., Das, S. & Hyman, B. T. APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 20 , 68–80 (2021).
Troutwine, B. R. et al. Apolipoprotein E and Alzheimer’s disease. Acta Pharm. Sin. B 12 , 496–510 (2022).
Rosenberg, R. N., Lambracht-Washington, D., Yu, G. & Xia, W. Genomics of Alzheimer Disease: A Review. JAMA Neurol. 73 , 867–874 (2016).
Nicolas, G. Recent advances in Alzheimer disease genetics. Curr. Opin. Neurol. 37 , 154–165 (2024).
Cline, E. N., Bicca, M. A., Viola, K. L. & Klein, W. L. The Amyloid-beta Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. 64 , S567–S610 (2018).
Giuffrida, M. L. et al. The monomer state of beta-amyloid: where the Alzheimer’s disease protein meets physiology. Rev. Neurosci. 21 , 83–93 (2010).
Kent, S. A., Spires-Jones, T. L. & Durrant, C. S. The physiological roles of tau and Abeta: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 140 , 417–447 (2020).
Zhang, Y. et al. Amyloid beta-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct. Target Ther. 8 , 248 (2023).
Cline, E. N., Bicca, M. A., Viola, K. L. & Klein, W. L. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. 64 , S567–S610 (2018).
Yu, H. & Wu, J. Amyloid-beta: A double agent in Alzheimer’s disease? Biomed. Pharmacother. 139 , 111575 (2021).
Lee, S. J. et al. Towards an understanding of amyloid-beta oligomers: characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 46 , 310–323 (2017).
Viles, J. H. Imaging Amyloid-β Membrane Interactions: Ion-Channel Pores and Lipid-Bilayer Permeability in Alzheimer’s Disease. Angew. Chem. Int. Ed. Engl. 62 , e202215785 (2023).
Salminen, A. et al. Inflammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog. Neurobiol. 87 , 181–194 (2009).
White, J. A. et al. Differential effects of oligomeric and fibrillar amyloid-beta 1-42 on astrocyte-mediated inflammation. Neurobiol. Dis. 18 , 459–465 (2005).
Demuro, A., Parker, I. & Stutzmann, G. E. Calcium signaling and amyloid toxicity in Alzheimer disease. J. Biol. Chem. 285 , 12463–12468 (2010).
Norambuena, A. et al. A novel lysosome-to-mitochondria signaling pathway disrupted by amyloid-β oligomers. EMBO J. 37 , e100241 (2018).
Reddy, P. H. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp. Neurol. 218 , 286–292 (2009).
Wang, X. et al. Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer disease. Free Radic. Biol. Med 43 , 1569–1573 (2007).
Butterfield, D. A., Swomley, A. M. & Sultana, R. Amyloid beta-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid. Redox Signal 19 , 823–835 (2013).
Wilcox, K. C., Lacor, P. N., Pitt, J. & Klein, W. L. Aβ oligomer-induced synapse degeneration in Alzheimer’s disease. Cell Mol. Neurobiol. 31 , 939–948 (2011).
Hardy, J. A. & Higgins, G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256 , 184–185 (1992).
Makin, S. The amyloid hypothesis on trial. Nature 559 , S4–S7 (2018).
Frisoni, G. B. et al. The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat. Rev. Neurosci. 23 , 53–66 (2022).
Granzotto, A. & Sensi, S. L. Once upon a time, the Amyloid Cascade Hypothesis. Ageing Res. Rev. 93 , 102161 (2024).
Morris, G. P., Clark, I. A. & Vissel, B. Questions concerning the role of amyloid-beta in the definition, aetiology and diagnosis of Alzheimer’s disease. Acta Neuropathol. 136 , 663–689 (2018).
Glass, D. J. & Arnold, S. E. Some evolutionary perspectives on Alzheimer’s disease pathogenesis and pathology. Alzheimers Dement . 8 , 343–351 (2012).
Roda, A. R. et al. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 17 , 1666–1674 (2022).
Zhang, H. et al. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int J. Biol. Sci. 17 , 2181–2192 (2021).
Bruni, A. C., Bernardi, L. & Gabelli, C. From beta amyloid to altered proteostasis in Alzheimer’s disease. Ageing Res. Rev. 64 , 101126 (2020).
Ossenkoppele, R., van der Kant, R. & Hansson, O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 21 , 726–734 (2022).
Sinsky, J., Pichlerova, K. & Hanes, J. Tau Protein Interaction Partners and Their Roles in Alzheimer’s Disease and Other Tauopathies. Int J. Mol. Sci. 22 , 9207 (2021).
Wei, Y., Liu, M. & Wang, D. The propagation mechanisms of extracellular tau in Alzheimer’s disease. J. Neurol. 269 , 1164–1181 (2022).
Tang, Y., Zhang, D., Gong, X. & Zheng, J. A mechanistic survey of Alzheimer’s disease. Biophys. Chem. 281 , 106735 (2022).
Chong, F. P., Ng, K. Y., Koh, R. Y. & Chye, S. M. Tau Proteins and Tauopathies in Alzheimer’s Disease. Cell Mol. Neurobiol. 38 , 965–980 (2018).
Bjørklund, G., Aaseth, J., Dadar, M. & Chirumbolo, S. Molecular Targets in Alzheimer’s Disease. Mol. Neurobiol. 56 , 7032–7044 (2019).
Almansoub, H. et al. Tau Abnormalities and the Potential Therapy in Alzheimer’s Disease. J. Alzheimers Dis. 67 , 13–33 (2019).
Wu, X. L. et al. Tau-mediated Neurodegeneration and potential implications in diagnosis and treatment of Alzheimer’s Disease. Chin. Med. J. 130 , 2978–2990 (2017).
Yin, X. et al. Dendritic/Post-synaptic Tau and Early Pathology of Alzheimer’s Disease. Front Mol. Neurosci. 14 , 671779 (2021).
Amadoro, G., Latina, V., Corsetti, V. & Calissano, P. N-terminal tau truncation in the pathogenesis of Alzheimer’s disease (AD): Developing a novel diagnostic and therapeutic approach. Biochim Biophys. Acta Mol. Basis Dis. 1866 , 165584 (2020).
Novak, M., Kabat, J. & Wischik, C. M. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer’s disease paired helical filament. EMBO J. 12 , 365–370 (1993).
Wang, J. Z., Grundke-Iqbal, I. & Iqbal, K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer’s disease. Nat. Med. 2 , 871–875 (1996).
Liu, K. et al. Glycation alter the process of Tau phosphorylation to change Tau isoforms aggregation property. Biochim. Biophys. Acta 1862 , 192–201 (2016).
Luo, H. B. et al. SUMOylation at K340 inhibits tau degradation through deregulating its phosphorylation and ubiquitination. Proc. Natl Acad. Sci. USA 111 , 16586–16591 (2014).
Ray, W. J. & Buggia-Prevot, V. Novel Targets for Alzheimer’s Disease: A View Beyond Amyloid. Annu. Rev. Med. 72 , 15–28 (2021).
Sintini, I. et al. Longitudinal rates of atrophy and tau accumulation differ between the visual and language variants of atypical Alzheimer’s disease. Alzheimers Dement. 19 , 4396–4406 (2023).
Whitwell, J. L. et al. Imaging correlations of tau, amyloid, metabolism, and atrophy in typical and atypical Alzheimer’s disease. Alzheimers Dement. 14 , 1005–1014 (2018).
Weston, P. S. J. et al. Cortical tau is associated with microstructural imaging biomarkers of neurite density and dendritic complexity in Alzheimer’s disease. Alzheimers Dement. 19 , 2750–2754 (2023).
Sala Frigerio, C. & De Strooper, B. Alzheimer’s Disease Mechanisms and Emerging Roads to Novel Therapeutics. Annu. Rev. Neurosci. 39 , 57–79 (2016).
Leng, F. & Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17 , 157–172 (2021).
von Bernhardi, R., Eugenín-von Bernhardi, L. & Eugenín, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci. 7 , 124 (2015).
Balducci, C. & Forloni, G. Novel targets in Alzheimer’s disease: A special focus on microglia. Pharm. Res. 130 , 402–413 (2018).
Hickman, S. E., Allison, E. K. & El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 28 , 8354–8360 (2008).
Ferrari, C. & Sorbi, S. The complexity of Alzheimer’s disease: an evolving puzzle. Physiol. Rev. 101 , 1047–1081 (2021).
Venegas, C. et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552 , 355–361 (2017).
Busche, M. A. & Hyman, B. T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 23 , 1183–1193 (2020).
Španić, E., Langer Horvat, L., Hof, P. R. & Šimić, G. Role of Microglial Cells in Alzheimer’s Disease Tau Propagation. Front. Aging Neurosci. 11 , 271 (2019).
Sumsuzzman, D. M. et al. Microglia in Alzheimer’s Disease: A Favorable Cellular Target to Ameliorate Alzheimer’s Pathogenesis. Mediators Inflamm. 2022 , 6052932 (2022).
Althafar, Z. M. Targeting Microglia in Alzheimer’s Disease: From Molecular Mechanisms to Potential Therapeutic Targets for Small Molecules. Molecules 27 , 4124 (2022).
Heneka, M. T. ApoE4 makes microglia trem(2)bling. Neuron 111 , 142–144 (2023).
Guo, T. et al. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 15 , 40 (2020).
Liu, C. C. et al. Cell-autonomous effects of APOE4 in restricting microglial response in brain homeostasis and Alzheimer’s disease. Nat. Immunol. 24 , 1854–1866 (2023).
Self, W. K. & Holtzman, D. M. Emerging diagnostics and therapeutics for Alzheimer disease. Nat. Med. 29 , 2187–2199 (2023).
Zhang, W., Xiao, D., Mao, Q. & Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target Ther. 8 , 267 (2023).
Nouraeinejad, A. The Link Between COVID-19 and Alzheimer Disease Through Neuroinflammation. Clin. Med. Res. 21 , 119–121 (2023).
Bello-Corral, L. et al. Implications of gut and oral microbiota in neuroinflammatory responses in Alzheimer’s disease. Life Sci. 333 , 122132 (2023).
Wong-Guerra, M., Calfio, C., Maccioni, R. B. & Rojo, L. E. Revisiting the neuroinflammation hypothesis in Alzheimer’s disease: a focus on the druggability of current targets. Front. Pharm. 14 , 1161850 (2023).
Calsolaro, V. & Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 12 , 719–732 (2016).
Lecca, D. et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis. Alzheimers Dement. 18 , 2327–2340 (2022).
Praticò, D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharm. Sci. 29 , 609–615 (2008).
Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 71 , 621S–629S (2000).
Markesbery, W. R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 23 , 134–147 (1997).
Miranda, S. et al. The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog. Neurobiol. 62 , 633–648 (2000).
Bai, R. et al. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 77 , 101619 (2022).
Chen, Z. & Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 30 , 271–281 (2014).
Rosini, M. et al. Oxidative stress in Alzheimer’s disease: are we connecting the dots? J. Med. Chem. 57 , 2821–2831 (2014).
Ferreira, M. E. et al. Oxidative Stress in Alzheimer’s Disease: Should We Keep Trying Antioxidant Therapies? Cell Mol. Neurobiol. 35 , 595–614 (2015).
Poprac, P. et al. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharm. Sci. 38 , 592–607 (2017).
Perluigi, M., Di Domenico, F. & Butterfield, D. A. Oxidative damage in neurodegeneration: roles in the pathogenesis and progression of Alzheimer disease. Physiol. Rev. 104 , 103–197 (2024).
Roy, R. G., Mandal, P. K. & Maroon, J. C. Oxidative Stress Occurs Prior to Amyloid Aβ Plaque Formation and Tau Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 14 , 2944–2954 (2023).
Sanders, O. D., Rajagopal, L. & Rajagopal, J. A. The oxidatively damaged DNA and amyloid-β oligomer hypothesis of Alzheimer’s disease. Free Radic. Biol. Med. 179 , 403–412 (2022).
Chen, L. L. et al. The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorg. Chem. 131 , 106301 (2023).
Ayton, S., Lei, P. & Bush, A. I. Metallostasis in Alzheimer’s disease. Free Radic. Biol. Med. 62 , 76–89 (2013).
Sensi, S. L., Granzotto, A., Siotto, M. & Squitti, R. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends Pharm. Sci. 39 , 1049–1063 (2018).
D’Acunto, C. W. et al. Metallomics for Alzheimer’s disease treatment: Use of new generation of chelators combining metal-cation binding and transport properties. Eur. J. Med. Chem. 150 , 140–155 (2018).
Caraci, F., Nicoletti, F. & Copani, A. Metabotropic glutamate receptors: the potential for therapeutic applications in Alzheimer’s disease. Curr. Opin. Pharm. 38 , 1–7 (2018).
Sharma, P. et al. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 174 , 53–89 (2019).
Zhong, W. et al. Pathogenesis of sporadic Alzheimer’s disease by deficiency of NMDA receptor subunit GluN3A. Alzheimers Dement. 18 , 222–239 (2022).
Verma, M., Lizama, B. N. & Chu, C. T. Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl. Neurodegener. 11 , 3 (2022).
Granzotto, A., Canzoniero, L. M. T. & Sensi, S. L. A Neurotoxic Ménage-à-trois: Glutamate, Calcium, and Zinc in the Excitotoxic Cascade. Front. Mol. Neurosci. 13 , 600089 (2020).
Bi, D., Wen, L., Wu, Z. & Shen, Y. GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer’s disease. Alzheimers Dement. 16 , 1312–1329 (2020).
Liu, S. et al. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 57 , 5026–5043 (2020).
Bulgart, H. R., Neczypor, E. W., Wold, L. E. & Mackos, A. R. Microbial involvement in Alzheimer disease development and progression. Mol. Neurodegener. 15 , 42 (2020).
La Rosa, F. et al. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients 10 , 1267 (2018).
Hu, X., Wang, T. & Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 59 , 1006–1023 (2016).
Sochocka, M. et al. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol. Neurobiol. 56 , 1841–1851 (2019).
Megur, A., Baltriukienė, D., Bukelskienė, V. & Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 13 , 37 (2020).
Ferreiro, A. L. et al. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl. Med . 15 , eabo2984 (2023).
Zhang, X.-W., Zhu, X.-X., Tang, D.-S. & Lu, J.-H. Targeting autophagy in Alzheimer’s disease: Animal models and mechanisms. Zool. Res. 44 , 1132–1145 (2023).
Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1 , 634–650 (2021).
Eshraghi, M. et al. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharm. Ther. 237 , 108171 (2022).
Kaushik, S. & Cuervo, A. M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19 , 365–381 (2018).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 17 , 1–382 (2021).
Nedelsky, N. B., Todd, P. K. & Taylor, J. P. Autophagy and the ubiquitin-proteasome system: Collaborators in neuroprotection. Biochim Biophys. Acta 1782 , 691–699 (2008).
Dong, Z. & Cui, H. The Autophagy-Lysosomal Pathways and Their Emerging Roles in Modulating Proteostasis in Tumors. Cells 8 , 4 (2018).
Deng, Z. et al. Pharmacological modulation of autophagy for Alzheimer’s disease therapy: Opportunities and obstacles. Acta Pharm. Sin. B 12 , 1688–1706 (2022).
Iranpour, M. et al. Apoptosis, autophagy and unfolded protein response pathways in Arbovirus replication and pathogenesis. Expert Rev. Mol. Med. 18 , e1 (2016).
Nixon, R. A. The role of autophagy in neurodegenerative disease. Nat. Med. 19 , 983–997 (2013).
Yu, W. H. et al. Macroautophagy—a novel β-amyloid peptide-generating pathway activated in Alzheimer’s disease. J. Cell Biol. 171 , 87–98 (2005).
Kerr, J. S. et al. Mitophagy and Alzheimer’s Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 40 , 151–166 (2017).
Bourdenx, M. et al. Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell 184 , 2696–2714.e2625 (2021).
Wang, Z. et al. Microglial autophagy in Alzheimer’s disease and Parkinson’s disease. Front. Aging Neurosci. 14 , 1065183 (2023).
Litwiniuk, A., Juszczak, G. R., Stankiewicz, A. M. & Urbańska, K. The role of glial autophagy in Alzheimer’s disease. Mol. Psychiatry 28 , 4528–4539 (2023).
Zhang, Z., Yang, X., Song, Y.-Q. & Tu, J. Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res. Rev. 72 , 101464 (2021).
Chandra, S. & Pahan, K. Gemfibrozil, a Lipid-Lowering Drug, Lowers Amyloid Plaque Pathology and Enhances Memory in a Mouse Model of Alzheimer’s Disease via Peroxisome Proliferator-Activated Receptor α. J. Alzheimers Dis. Rep. 3 , 149–168 (2019).
Luo, R. et al. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy 16 , 52–69 (2020).
Zheng, Y. et al. Inflammatory signaling pathways in the treatment of Alzheimer’s disease with inhibitors, natural products and metabolites (Review). Int. J. Mol. Med. 52 , 111 (2023).
Thakur, S. et al. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 46 , 1–17 (2023).
Dhapola, R. et al. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 29 , 1669–1681 (2021).
Li, Z. et al. Targeting protein kinases for the treatment of Alzheimer’s disease: Recent progress and future perspectives. Eur. J. Med. Chem. 261 , 115817 (2023).
Huang, R. et al. Whole-plant foods and their macromolecules: untapped approaches to modulate neuroinflammation in Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 63 , 2388–2406 (2023).
Seo, E.-J., Fischer, N. & Efferth, T. J. P. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Pharm. Res. 129 , 262–273 (2018).
Morgan, M. J. & Liu, Z. G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21 , 103–115 (2011).
Zhao, K. et al. The miR-25802/KLF4/NF-κB signaling axis regulates microglia-mediated neuroinflammation in Alzheimer’s disease. Brain Behav. Immun. 118 , 31–48 (2024).
Blevins, H. M., Xu, Y., Biby, S. & Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front Aging Neurosci. 14 , 879021 (2022).
Naeem, A. et al. MCC950 reduces autophagy and improves cognitive function by inhibiting NLRP3-dependent neuroinflammation in a rat model of Alzheimer’s disease. Brain Behav. Immun. 116 , 70–84 (2024).
Terzioglu, G. & Young-Pearse, T. L. Microglial function, INPP5D/SHIP1 signaling, and NLRP3 inflammasome activation: implications for Alzheimer’s disease. Mol. Neurodegener. 18 , 89 (2023).
Moonen, S. et al. Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol. 145 , 175–195 (2023).
Wang, C. et al. Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nat. Commun. 13 , 1969 (2022).
Dutta, D. et al. Tau fibrils induce glial inflammation and neuropathology via TLR2 in Alzheimer’s disease-related mouse models. J. Clin. Invest. 133 , e161987 (2023).
Ising, C. et al. NLRP3 inflammasome activation drives tau pathology. Nature 575 , 669–673 (2019).
Xie, X. et al. Activation of innate immune cGAS-STING pathway contributes to Alzheimer’s pathogenesis in 5×FAD mice. Nat. Aging 3 , 202–212 (2023).
Huang, Y. et al. Mechanism and therapeutic potential of targeting cGAS-STING signaling in neurological disorders. Mol. Neurodegener. 18 , 79 (2023).
Jorfi, M., Maaser-Hecker, A. & Tanzi, R. E. The neuroimmune axis of Alzheimer’s disease. Genome Med. 15 , 6 (2023).
Dionisio-Santos, D. A., Olschowka, J. A. & O’Banion, M. K. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J. Neuroinflamm. 16 , 74 (2019).
Jorfi, M. et al. Infiltrating CD8+ T cells exacerbate Alzheimer’s disease pathology in a 3D human neuroimmune axis model. Nat. Neurosci. 26 , 1489–1504 (2023).
Unger, M. S. et al. CD8(+) T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav. Immun. 89 , 67–86 (2020).
Wang, Y. et al. TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice. Aging 12 , 20862–20879 (2020).
Zhong, L. et al. TREM2 receptor protects against complement-mediated synaptic loss by binding to complement C1q during neurodegeneration. Immunity 56 , 1794–1808.e8 (2023).
Cao, M., Luo, X., Wu, K. & He, X. Targeting lysosomes in human disease: from basic research to clinical applications. Signal Transduct. Target Ther. 6 , 379 (2021).
Hu, Y. B., Dammer, E. B., Ren, R. J. & Wang, G. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 4 , 18 (2015).
Orr, M. E. & Oddo, S. Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res. Ther. 5 , 53 (2013).
Xiong, J. & Zhu, M. X. Regulation of lysosomal ion homeostasis by channels and transporters. Sci. China Life Sci. 59 , 777–791 (2016).
Lo, C. H. & Zeng, J. Defective lysosomal acidification: a new prognostic marker and therapeutic target for neurodegenerative diseases. Transl. Neurodegener. 12 , 29 (2023).
Lee, J. H. et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141 , 1146–1158 (2010).
Zhang, X. et al. A role for presenilins in autophagy revisited: normal acidification of lysosomes in cells lacking PSEN1 and PSEN2. J. Neurosci. 32 , 8633–8648 (2012).
Coen, K. et al. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 198 , 23–35 (2012).
Lee, J. H. et al. Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep. 12 , 1430–1444 (2015).
Tong, B. C. et al. Lysosomal TPCN (two pore segment channel) inhibition ameliorates beta-amyloid pathology and mitigates memory impairment in Alzheimer disease. Autophagy 18 , 624–642 (2022).
Im, E. et al. Lysosomal dysfunction in Down syndrome and Alzheimer mouse models is caused by v-ATPase inhibition by Tyr(682)-phosphorylated APP βCTF. Sci. Adv. 9 , eadg1925 (2023).
Dietschy, J. M. & Turley, S. D. Cholesterol metabolism in the brain. Curr. Opin. Lipido 12 , 105–112 (2001).
Ahmed, H. et al. Brain cholesterol and Alzheimer’s disease: challenges and opportunities in probe and drug development. Brain 147 , 1622–1635 (2024).
Qian, L., Chai, A. B., Gelissen, I. C. & Brown, A. J. J. Eo. N. T. Balancing cholesterol in the brain: From synthesis to disposal. Explor Neuroprot. Ther. 2 , 1–27 (2022).
Li, D., Zhang, J. & Liu, Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 45 , 401–414 (2022).
Feringa, F. M. & van der Kant, R. Cholesterol and Alzheimer’s Disease; From Risk Genes to Pathological Effects. Front Aging Neurosci. 13 , 690372 (2021).
Wolozin, B. Cholesterol and the biology of Alzheimer’s disease. Neuron 41 , 7–10 (2004).
Wang, H. et al. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl Acad. Sci. USA 118 , e2102191118 (2021).
Huang, S. et al. Chimeric cerebral organoids reveal the essentials of neuronal and astrocytic APOE4 for Alzheimer’s tau pathology. Signal Transduct. Target Ther. 7 , 176 (2022).
Litvinchuk, A. et al. Amelioration of Tau and ApoE4-linked glial lipid accumulation and neurodegeneration with an LXR agonist. Neuron 112 , 384–403.e8 (2024).
de Dios, C. et al. Inflammasome activation under high cholesterol load triggers a protective microglial phenotype while promoting neuronal pyroptosis. Transl. Neurodegener. 12 , 10 (2023).
Gowda, P., Reddy, P. H. & Kumar, S. Deregulated mitochondrial microRNAs in Alzheimer’s disease: Focus on synapse and mitochondria. Ageing Res. Rev. 73 , 101529 (2022).
Wang, W. et al. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol. Neurodegener. 15 , 30 (2020).
Kapogiannis, D. & Mattson, M. P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 10 , 187–198 (2011).
Godoy, J. A. et al. Signaling pathway cross talk in Alzheimer’s disease. Cell Commun. Signal 12 , 23 (2014).
Eckert, A., Schmitt, K. & Götz, J. Mitochondrial dysfunction - the beginning of the end in Alzheimer’s disease? Separate and synergistic modes of tau and amyloid-β toxicity. Alzheimers Res. Ther. 3 , 15 (2011).
Lustbader, J. W. et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 304 , 448–452 (2004).
Ye, Z. et al. Aβ-binding with alcohol dehydrogenase drives Alzheimer’s disease pathogenesis: A review. Int. J. Biol. Macromol. 264 , 130580 (2024).
Misrani, A., Tabassum, S. & Yang, L. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front. Aging Neurosci. 13 , 57 (2021).
De Nicolo, B., Cataldi-Stagetti, E., Diquigiovanni, C. & Bonora, E. Calcium and Reactive Oxygen Species Signaling Interplays in Cardiac Physiology and Pathologies. Antioxidants 12 , 353 (2023).
Bezprozvanny, I. & Mattson, M. P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 31 , 454–463 (2008).
Resende, R., Ferreiro, E., Pereira, C. & Resende de Oliveira, C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1-42: involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience 155 , 725–737 (2008).
Calvo-Rodriguez, M. & Bacskai, B. J. High mitochondrial calcium levels precede neuronal death in vivo in Alzheimer’s disease. Cell Stress 4 , 187–190 (2020).
Calvo-Rodriguez, M. et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 11 , 2146 (2020).
Du, H. & ShiDu Yan, S. Unlocking the Door to Neuronal Woes in Alzheimer’s Disease: Aβ and Mitochondrial Permeability Transition Pore. Pharmaceuticals 3 , 1936–1948 (2010).
Calvo-Rodriguez, M. & Bacskai, B. J. Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death. Trends Neurosci. 44 , 136–151 (2021).
Millecamps, S. & Julien, J. P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 14 , 161–176 (2013).
Chen, W., Zhao, H. & Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct. Target Ther. 8 , 333 (2023).
Cho, D. H. et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324 , 102–105 (2009).
Bossy, B. et al. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. J. Alzheimers Dis. 20 (Suppl 2), S513–526 (2010).
Flannery, P. J. & Trushina, E. Mitochondrial dynamics and transport in Alzheimer’s disease. Mol. Cell Neurosci. 98 , 109–120 (2019).
Pradeepkiran, J. A. & Reddy, P. H. Defective mitophagy in Alzheimer’s disease. Ageing Res. Rev. 64 , 101191 (2020).
John, A. & Reddy, P. H. Synaptic basis of Alzheimer’s disease: Focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res. Rev. 65 , 101208 (2021).
Li, X. et al. Molecular mechanisms of mitophagy and its roles in neurodegenerative diseases. Pharm. Res. 163 , 105240 (2021).
Zeng, K. et al. Defective mitophagy and the etiopathogenesis of Alzheimer’s disease. Transl. Neurodegener. 11 , 32 (2022).
Mary, A., Eysert, F., Checler, F. & Chami, M. Mitophagy in Alzheimer’s disease: Molecular defects and therapeutic approaches. Mol. Psychiatry 28 , 202–216 (2023).
Webber, E. K., Fivaz, M., Stutzmann, G. E. & Griffioen, G. Cytosolic calcium: Judge, jury and executioner of neurodegeneration in Alzheimer’s disease and beyond. Alzheimers Dement . 19 , 3701–3717 (2023).
McDaid, J., Mustaly-Kalimi, S. & Stutzmann, G. E. Ca(2+) Dyshomeostasis Disrupts Neuronal and Synaptic Function in Alzheimer’s Disease. Cells 9 , 2655 (2020).
Pedriali, G. et al. Regulation of Endoplasmic Reticulum-Mitochondria Ca(2+) Transfer and Its Importance for Anti-Cancer Therapies. Front. Oncol. 7 , 180 (2017).
Kawamoto, E. M., Vivar, C. & Camandola, S. Physiology and pathology of calcium signaling in the brain. Front. Pharm. 3 , 61 (2012).
Madreiter-Sokolowski, C. T., Thomas, C. & Ristow, M. Interrelation between ROS and Ca(2+) in aging and age-related diseases. Redox Biol. 36 , 101678 (2020).
Baracaldo-Santamaría, D. et al. Role of Calcium Modulation in the Pathophysiology and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 24 , 9067 (2023).
Mochida, S. Calcium Channels and Calcium-Binding Proteins. Int J. Mol. Sci. 24 , 14257 (2023).
Mattson, M. P. Calcium and neurodegeneration. Aging Cell 6 , 337–350 (2007).
Bezprozvanny, I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 15 , 89–100 (2009).
Giorgi, C. et al. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 28 , 258–273 (2018).
Smajilovic, S. & Tfelt-Hansen, J. Calcium acts as a first messenger through the calcium-sensing receptor in the cardiovascular system. Cardiovasc. Res . 75 , 457–467 (2007).
Ozcan, L. & Tabas, I. Calcium signalling and ER stress in insulin resistance and atherosclerosis. J. Intern. Med. 280 , 457–464 (2016).
Crossley, C. A., Rajani, V. & Yuan, Q. Modulation of L-type calcium channels in Alzheimer’s disease: A potential therapeutic target. Comput Struct. Biotechnol. J. 21 , 11–20 (2023).
Anekonda, T. S. et al. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 41 , 62–70 (2011).
Tu, S., Okamoto, S., Lipton, S. A. & Xu, H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol. Neurodegener. 9 , 48 (2014).
Li, S. & Stern, A. M. Bioactive human Alzheimer brain soluble Aβ: pathophysiology and therapeutic opportunities. Mol. Psychiatry 27 , 3182–3191 (2022).
Uddin, M. S., Yu, W. S. & Lim, L. W. Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer’s disease. Ageing Res. Rev. 70 , 101417 (2021).
Carvalho, E. J., Stathopulos, P. B. & Madesh, M. Regulation of Ca(2+) exchanges and signaling in mitochondria. Curr. Opin. Physiol. 17 , 197–206 (2020).
Liu, Y. & Zhu, X. Endoplasmic reticulum-mitochondria tethering in neurodegenerative diseases. Transl. Neurodegener. 6 , 21 (2017).
Liu, J. & Yang, J. Mitochondria-associated membranes: A hub for neurodegenerative diseases. Biomed. Pharmacother. 149 , 112890 (2022).
Hedskog, L. et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc. Natl Acad. Sci. USA 110 , 7916–7921 (2013).
Area-Gomez, E. et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 31 , 4106–4123 (2012).
Yu, W., Jin, H. & Huang, Y. Mitochondria-associated membranes (MAMs): a potential therapeutic target for treating Alzheimer’s disease. Clin. Sci. 135 , 109–126 (2021).
Mustaly-Kalimi, S. et al. Protein mishandling and impaired lysosomal proteolysis generated through calcium dysregulation in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 119 , e2211999119 (2022).
De Felice, F. G., Gonçalves, R. A. & Ferreira, S. T. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat. Rev. Neurosci. 23 , 215–230 (2022).
Nowell, J., Blunt, E. & Edison, P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease. Mol. Psychiatry 28 , 217–229 (2023).
Correia, S. C. et al. Insulin-resistant brain state: the culprit in sporadic Alzheimer’s disease? Ageing Res. Rev. 10 , 264–273 (2011).
Nowell, J., Blunt, E., Gupta, D. & Edison, P. Antidiabetic agents as a novel treatment for Alzheimer’s and Parkinson’s disease. Ageing Res. Rev. 89 , 101979 (2023).
Kellar, D. & Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 19 , 758–766 (2020).
Steen, E. et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J. Alzheimers Dis. 7 , 63–80 (2005).
Gabbouj, S. et al. Altered Insulin Signaling in Alzheimer’s Disease Brain - Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 13 , 629 (2019).
Hooper, C., Killick, R. & Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 104 , 1433–1439 (2008).
Kim, B. & Feldman, E. L. Insulin resistance in the nervous system. Trends Endocrinol. Metab. 23 , 133–141 (2012).
Querfurth, H. & Lee, H. K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol. Neurodegener. 16 , 44 (2021).
Bomfim, T. R. et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J. Clin. Invest. 122 , 1339–1353 (2012).
Nagahara, A. H. & Tuszynski, M. H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 10 , 209–219 (2011).
Kim, J., He, M. J., Widmann, A. K. & Lee, F. S. The role of neurotrophic factors in novel, rapid psychiatric treatments. Neuropsychopharmacology 49 , 227–245 (2024).
Pentz, R. et al. The human brain NGF metabolic pathway is impaired in the pre-clinical and clinical continuum of Alzheimers disease. Mol. Psychiatry 26 , 6023–6037 (2021).
Triaca, V. et al. NGF controls APP cleavage by downregulating APP phosphorylation at Thr668: relevance for Alzheimer’s disease. Aging Cell 15 , 661–672 (2016).
Xhima, K. et al. Ultrasound delivery of a TrkA agonist confers neuroprotection to Alzheimer-associated pathologies. Brain 145 , 2806–2822 (2022).
Ding, X. W. et al. Nerve growth factor in metabolic complications and Alzheimer’s disease: Physiology and therapeutic potential. Biochim. Biophys. Acta Mol. Basis Dis. 1866 , 165858 (2020).
Phillips, H. S. et al. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 7 , 695–702 (1991).
Xiang, J. et al. Delta-secretase-cleaved Tau antagonizes TrkB neurotrophic signalings, mediating Alzheimer’s disease pathologies. Proc. Natl Acad. Sci. USA 116 , 9094–9102 (2019).
Wu, Z. et al. Neurotrophic signaling deficiency exacerbates environmental risks for Alzheimer’s disease pathogenesis. Proc. Natl Acad. Sci. USA 118 , e2100986118 (2021).
Wang, Z. H. et al. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 28 , 655–669.e5 (2019).
Matrone, C. et al. NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. Proc. Natl Acad. Sci. USA 105 , 13139–13144 (2008).
Tong, L., Balazs, R., Thornton, P. L. & Cotman, C. W. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J. Neurosci. 24 , 6799–6809 (2004).
Wang, W. et al. Microglial repopulation reverses cognitive and synaptic deficits in an Alzheimer’s disease model by restoring BDNF signaling. Brain Behav. Immun. 113 , 275–288 (2023).
Alkhalifa, A. E. et al. Blood-Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int J. Mol. Sci. 24 , 16288 (2023).
Nehra, G., Bauer, B. & Hartz, A. M. S. Blood-brain barrier leakage in Alzheimer’s disease: From discovery to clinical relevance. Pharm. Ther. 234 , 108119 (2022).
Chen, L. et al. Opportunities and challenges in delivering biologics for Alzheimer’s disease by low-intensity ultrasound. Adv. Drug Deliv. Rev. 189 , 114517 (2022).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12 , 723–738 (2011).
Bell, A. H., Miller, S. L., Castillo-Melendez, M. & Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 13 , 1452 (2019).
Montagne, A., Zhao, Z. & Zlokovic, B. V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 214 , 3151–3169 (2017).
Pan, Y. & Nicolazzo, J. A. Impact of aging, Alzheimer’s disease and Parkinson’s disease on the blood-brain barrier transport of therapeutics. Adv. Drug Deliv. Rev. 135 , 62–74 (2018).
Zenaro, E., Piacentino, G. & Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 107 , 41–56 (2017).
Erickson, M. A. & Banks, W. A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 33 , 1500–1513, (2013).
Winkler, E. A. et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 18 , 521–530 (2015).
Jack, C. R. et al. NIA‐AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14 , 535–562 (2018).
Mahaman, Y. A. R. et al. Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention. Ageing Res. Rev. 74 , 101544 (2022).
Scheltens, P. et al. Alzheimer’s disease. Lancet 397 , 1577–1590 (2021).
Klyucherev, T. O. et al. Advances in the development of new biomarkers for Alzheimer’s disease. Transl. Neurodegener. 11 , 25 (2022).
Brown RK, B. N.Wong, K. K., Minoshima, S. & Frey, K. A. Brain PET in suspected dementia: patterns of altered FDG metabolism. Radiographics 34 , 684–701 (2014).
van Oostveen, W. M. & de Lange, E. C. M. Imaging Techniques in Alzheimer’s Disease: A Review of Applications in Early Diagnosis and Longitudinal Monitoring. Int J. Mol. Sci. 22 , 2110 (2021).
Andersen, E. et al. Diagnostic biomarkers in Alzheimer’s disease. Biomark. Neuropsychiatry 5 , 100041 (2021).
Olsson, B. et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 15 , 673–684 (2016).
Dubois, B. et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13 , 614–629 (2014).
Bucci, M., Chiotis, K. & Nordberg, A. Alzheimer’s disease profiled by fluid and imaging markers: tau PET best predicts cognitive decline. Mol. Psychiatry 26 , 5888–5898 (2021).
Jia, J. et al. Biomarker Changes during 20 Years Preceding Alzheimer’s Disease. N. Engl. J. Med . 390 , 712–722 (2024).
Zhao, A. et al. Soluble TREM2 levels associate with conversion from mild cognitive impairment to Alzheimer’s disease. J. Clin. Invest . 132 , e158708 (2022).
Zetterberg, H. Biofluid‐based biomarkers for Alzheimer’s disease–related pathologies: An update and synthesis of the literature. Alzheimers Dement. 18 , 1687–1693 (2022).
Carter, S. F. et al. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol. Med. 25 , 77–95 (2019).
Kumar, A. et al. Amyloid and Tau in Alzheimer’s Disease: Biomarkers or Molecular Targets for Therapy? Are We Shooting the Messenger? Am. J. Psychiatry 178 , 1014–1025 (2021).
Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25 , 270–276 (2019).
Wong, M. W. et al. Dysregulation of lipids in Alzheimer’s disease and their role as potential biomarkers. Alzheimers Dement. 13 , 810–827 (2017).
Paciotti, S. et al. Potential diagnostic value of CSF metabolism-related proteins across the Alzheimer’s disease continuum. Alzheimers Res. Ther. 15 , 124 (2023).
Yuyama, K. et al. Extracellular vesicle proteome unveils cathepsin B connection to Alzheimer’s disease pathogenesis. Brain 147 , 627–636 (2023).
Article PubMed Central Google Scholar
Chatterjee, M. et al. C1q is increased in cerebrospinal fluid‐derived extracellular vesicles in Alzheimer’s disease: A multi‐cohort proteomics and immuno‐assay validation study. Alzheimers Dement. 19 , 4828–4840 (2023).
Blennow, K. et al. The potential clinical value of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement. 19 , 5805–5816 (2023).
Rani, S. et al. Advanced Overview of Biomarkers and Techniques for Early Diagnosis of Alzheimer’s Disease. Cell Mol. Neurobiol. 43 , 2491–2523 (2023).
Hu, S., Yang, C. & Luo, H. Current trends in blood biomarker detection and imaging for Alzheimer’s disease. Biosens. Bioelectron. 210 , 114278 (2022).
Teunissen, C. E. et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 21 , 66–77 (2022).
Blennow, K. & Zetterberg, H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J. Intern. Med. 284 , 643–663 (2018).
Jiang, Y. et al. A blood-based multi-pathway biomarker assay for early detection and staging of Alzheimer’s disease across ethnic groups. Alzheimers Dement . 20 , 2000–2015 (2024).
Valenza, M. & Scuderi, C. How useful are biomarkers for the diagnosis of Alzheimer’s disease and especially for its therapy? Neural Regen. Res. 17 , 2205–2207 (2022).
Schindler, S. E. & Karikari, T. K. Comorbidities confound Alzheimer’s blood tests. Nat. Med. 28 , 1349–1351 (2022).
O’Bryant, S. E., Petersen, M., Hall, J. & Johnson, L. A. Medical comorbidities and ethnicity impact plasma Alzheimer’s disease biomarkers: Important considerations for clinical trials and practice. Alzheimers Dement. 19 , 36–43 (2023).
Cousins, K. A. Q. et al. CSF Biomarkers of Alzheimer Disease in Patients With Concomitant α-Synuclein Pathology. Neurology 99 , e2303–e2312 (2022).
Mielke, M. M. et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat. Med. 28 , 1398–1405 (2022).
Galimberti, D. & Scarpini, E. Old and new acetylcholinesterase inhibitors for Alzheimer’s disease. Expert Opin. Investig. Drugs 25 , 1181–1187 (2016).
Masters, C. L. et al. Alzheimer’s disease. Nat. Rev. Dis. Prim. 1 , 15056 (2015).
Akıncıoğlu, H. & Gülçin, İ. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini Rev. Med. Chem. 20 , 703–715 (2020).
Simunkova, M. et al. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 93 , 2491–2513 (2019).
Thompson, S., Lanctôt, K. L. & Herrmann, N. The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer’s disease. Expert Opin. Drug Saf. 3 , 425–440 (2004).
Nunes, D., Loureiro, J. A. & Pereira, M. C. Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer’s. Drugs Pharm. 14 , 2296 (2022).
CAS Google Scholar
Kabir, M. T. et al. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 21 , 3272 (2020).
Joe, E. & Ringman, J. M. Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ 367 , l6217 (2019).
Larkin, H. D. First Donepezil Transdermal Patch Approved for Alzheimer Disease. JAMA 327 , 1642 (2022).
Tariot, P. N., Braeckman, R. & Oh, C. Comparison of Steady-State Pharmacokinetics of Donepezil Transdermal Delivery System with Oral Donepezil. J. Alzheimers Dis. 90 , 161–172 (2022).
Taléns-Visconti, R. et al. Intranasal Drug Administration in Alzheimer-Type Dementia: Towards Clinical Applications. Pharmaceutics 15 , 1399 (2023).
Georgieva, D., Nikolova, D., Vassileva, E. & Kostova, B. Chitosan-Based Nanoparticles for Targeted Nasal Galantamine Delivery as a Promising Tool in Alzheimer’s Disease Therapy. Pharmaceutics 15 , 829 (2023).
Amat-Ur-Rasool, H., Ahmed, M., Hasnain, S. & Carter, W. G. Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer’s Disease. Brain Sci. 11 , 184 (2021).
Miculas, D. C. et al. Pharmacotherapy Evolution in Alzheimer’s Disease: Current Framework and Relevant Directions. Cells 12 , 131 (2022).
Pardo-Moreno, T. et al. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 14 , 1117 (2022).
Huang, L. K., Chao, S. P. & Hu, C. J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 27 , 18 (2020).
Cummings, J. & Fox, N. Defining Disease Modifying Therapy for Alzheimer’s Disease. J. Prev. Alzheimers Dis. 4 , 109–115 (2017).
CAS PubMed PubMed Central Google Scholar
Yeo-Teh, N. S. L. & Tang, B. L. A Review of Scientific Ethics Issues Associated with the Recently Approved Drugs for Alzheimer’s Disease. Sci. Eng. Ethics 29 , 2 (2023).
Wang, X. et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 29 , 787–803 (2019).
Bosch, M. E. et al. Sodium oligomannate alters gut microbiota, reduces cerebral amyloidosis and reactive microglia in a sex-specific manner. Mol. Neurodegener. 19 , 18 (2024).
Liu, K. Y. & Howard, R. Can we learn lessons from the FDA’s approval of aducanumab? Nat. Rev. Neurol. 17 , 715–722 (2021).
Lythgoe, M. P., Jenei, K. & Prasad, V. Regulatory decisions diverge over aducanumab for Alzheimer’s disease. BMJ 376 , e069780 (2022).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537 , 50–56 (2016).
Karran, E. & De Strooper, B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov. 21 , 306–318 (2022).
Jucker, M. & Walker, L. C. Alzheimer’s disease: From immunotherapy to immunoprevention. Cell 186 , 4260–4270 (2023).
Lannfelt, L. et al. BAN2401 shows stronger binding to soluble aggregated amyloid-beta species than aducanumab. Alzheimers Dement. 15 , P1601–P1602 (2019).
Golde, T. E. & Levey, A. I. Immunotherapies for Alzheimer’s disease. Science 382 , 1242–1244 (2023).
Boxer, A. L. & Sperling, R. Accelerating Alzheimer’s therapeutic development: The past and future of clinical trials. Cell 186 , 4757–4772 (2023).
Couzin-Frankel, J. Side effects loom over Alzheimer’s drugs. Science 381 , 466–467 (2023).
Cao, Y., Yu, F., Lyu, Y. & Lu, X. Promising candidates from drug clinical trials: Implications for clinical treatment of Alzheimer’s disease in China. Front. Neurol. 13 , 1034243 (2022).
Ballard, C. Brexpiprazole for the Treatment of Agitation and Aggression in Alzheimer Disease. JAMA Neurol. 80 , 1272–1273 (2023).
Almeida, O. P. & Ford, A. H. Are We Getting Better at Managing Agitation in Dementia? Am. J. Geriatr. Psychiatry 28 , 401–403 (2020).
Bauzon, J., Lee, G. & Cummings, J. Repurposed agents in the Alzheimer’s disease drug development pipeline. Alzheimers Res. Ther. 12 , 98 (2020).
Grabowska, M. E. et al. Drug repurposing for Alzheimer’s disease from 2012-2022-a 10-year literature review. Front. Pharm. 14 , 1257700 (2023).
Ballard, C. et al. Drug repositioning and repurposing for Alzheimer disease. Nat. Rev. Neurol. 16 , 661–673 (2020).
Zang, C. et al. High-throughput target trial emulation for Alzheimer’s disease drug repurposing with real-world data. Nat. Commun. 14 , 8180 (2023).
Rodriguez, S. et al. Machine learning identifies candidates for drug repurposing in Alzheimer’s disease. Nat. Commun. 12 , 1033 (2021).
Tsuji, S. et al. Artificial intelligence-based computational framework for drug-target prioritization and inference of novel repositionable drugs for Alzheimer’s disease. Alzheimers Res. Ther. 13 , 92 (2021).
Cummings, J. et al. Alzheimer’s disease drug development pipeline: 2023. Alzheimers Dement. 9 , e12385 (2023).
Google Scholar
van Bokhoven, P. et al. The Alzheimer’s disease drug development landscape. Alzheimers Res. Ther. 13 , 186 (2021).
Elmaleh, D. R. et al. Developing Effective Alzheimer’s Disease Therapies: Clinical Experience and Future Directions. J. Alzheimers Dis. 71 , 715–732 (2019).
Zhang, F. et al. New therapeutics beyond amyloid-β and tau for the treatment of Alzheimer’s disease. Acta Pharm. Sin. 42 , 1382–1389 (2021).
Doody, R. S. et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 369 , 341–350 (2013).
Blennow, K., Zetterberg, H., Haass, C. & Finucane, T. Semagacestat’s fall: where next for AD therapies? Nat. Med. 19 , 1214–1215 (2013).
Yang, G. et al. Structural basis of γ-secretase inhibition and modulation by small molecule drugs. Cell 184 , 521–533.e14 (2021).
Gupta, V. B., Gupta, V. K. & Martins, R. Semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 369 , 1660–1661, (2013).
Coric, V. et al. Targeting Prodromal Alzheimer Disease With Avagacestat: A Randomized Clinical Trial. JAMA Neurol. 72 , 1324–1333 (2015).
Gravitz, L. Drugs: a tangled web of targets. Nature 475 , S9–11 (2011).
Hrabinova, M. et al. Is It the Twilight of BACE1 Inhibitors? Curr. Neuropharmacol. 19 , 61–77 (2021).
Neumann, U. et al. The BACE-1 inhibitor CNP520 for prevention trials in Alzheimer’s disease. EMBO Mol. Med. 10 , e9316 (2018).
Jeremic, D., Jiménez-Díaz, L. & Navarro-López, J. D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: a systematic review. Ageing Res. Rev. 72 , 101496 (2021).
Walsh, T. et al. Outreach, Screening, and Randomization of APOE ε4 Carriers into an Alzheimer’s Prevention Trial: A global Perspective from the API Generation Program. J. Prev. Alzheimers Dis. 10 , 453–463 (2023).
Imbimbo, B. P. & Watling, M. Investigational BACE inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 28 , 967–975 (2019).
Navarro-Gómez, N., Valdes-Gonzalez, M., Garrido-Suárez, B. B. & Garrido, G. Pharmacological Inventions for Alzheimer Treatment in the United States of America: A Revision Patent from 2010–2020. J. Prev. Alzheimers Dis. 10 , 50–68 (2023).
Tolar, M. et al. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res. Ther. 12 , 95 (2020).
Tolar, M., Abushakra, S. & Sabbagh, M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 16 , 1553–1560 (2020).
Hoffmann, T. et al. Combination of the Glutaminyl Cyclase Inhibitor PQ912 (Varoglutamstat) and the Murine Monoclonal Antibody PBD-C06 (m6) Shows Additive Effects on Brain Aβ Pathology in Transgenic Mice. Int J. Mol. Sci. 22 , 11791 (2021).
Van Manh, N. et al. Discovery of potent indazole-based human glutaminyl cyclase (QC) inhibitors as Anti-Alzheimer’s disease agents. Eur. J. Med. Chem. 244 , 114837 (2022).
Scheltens, P. et al. Safety, tolerability and efficacy of the glutaminyl cyclase inhibitor PQ912 in Alzheimer’s disease: results of a randomized, double-blind, placebo-controlled phase 2a study. Alzheimers Res. Ther. 10 , 107 (2018).
Vijverberg, E. G. B. et al. Rationale and study design of a randomized, placebo-controlled, double-blind phase 2b trial to evaluate efficacy, safety, and tolerability of an oral glutaminyl cyclase inhibitor varoglutamstat (PQ912) in study participants with MCI and mild AD-VIVIAD. Alzheimers Res. Ther. 13 , 142 (2021).
The, L. Alzheimer’s disease: expedition into the unknown. Lancet . 388 , 2713 (2016).
Sacks, C. A., Avorn, J. & Kesselheim, A. S. The Failure of Solanezumab - How the FDA Saved Taxpayers Billions. N. Engl. J. Med. 376 , 1706–1708 (2017).
Karran, E. & Hardy, J. Antiamyloid therapy for Alzheimer’s disease–are we on the right road? N. Engl. J. Med. 370 , 377–378 (2014).
Honig, L. S. et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 378 , 321–330 (2018).
Bateman, R. J. et al. Gantenerumab: an anti-amyloid monoclonal antibody with potential disease-modifying effects in early Alzheimer’s disease. Alzheimers Res. Ther. 14 , 178 (2022).
Bateman, R. J. et al. Two Phase 3 Trials of Gantenerumab in Early Alzheimer’s Disease. N. Engl. J. Med. 389 , 1862–1876 (2023).
Schneider, L. S. What the Gantenerumab Trials Teach Us about Alzheimer’s Treatment. N. Engl. J. Med. 389 , 1918–1920 (2023).
Basheer, N. et al. Does modulation of tau hyperphosphorylation represent a reasonable therapeutic strategy for Alzheimer’s disease? From preclinical studies to the clinical trials. Mol. Psychiatry 28 , 2197–2214 (2023).
Dong, Y. et al. Design, synthesis and bioevaluation of 1,2,4-thiadiazolidine-3,5-dione derivatives as potential GSK-3beta inhibitors for the treatment of Alzheimer’s disease. Bioorg. Chem. 134 , 106446 (2023).
Gulisano, W. et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimers Dis. 64 , S611–S631 (2018).
Wilcock, G. K. et al. Potential of Low Dose Leuco-Methylthioninium Bis(Hydromethanesulphonate) (LMTM) Monotherapy for Treatment of Mild Alzheimer’s Disease: Cohort Analysis as Modified Primary Outcome in a Phase III Clinical Trial. J. Alzheimers Dis. 61 , 435–457 (2018).
Gauthier, S. et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388 , 2873–2884 (2016).
Wischik, C. M. et al. Oral Tau Aggregation Inhibitor for Alzheimer’s Disease: Design, Progress and Basis for Selection of the 16 mg/day Dose in a Phase 3, Randomized, Placebo-Controlled Trial of Hydromethylthionine Mesylate. J. Prev. Alzheimers Dis. 9 , 780–790 (2022).
Panza, F. et al. Clinical development of passive tau-based immunotherapeutics for treating primary and secondary tauopathies. Expert Opin. Investig. Drugs 32 , 625–634 (2023).
Roberts, M. et al. Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer’s disease. Acta Neuropathol. Commun. 8 , 13 (2020).
Novak, P. et al. ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat. Aging 1 , 521–534 (2021).
Reading, C. L., Ahlem, C. N. & Murphy, M. F. NM101 Phase III study of NE3107 in Alzheimer’s disease: rationale, design and therapeutic modulation of neuroinflammation and insulin resistance. Neurodegener. Dis. Manag. 11 , 289–298 (2021).
Lozupone, M. et al. Anti-amyloid-β protein agents for the treatment of Alzheimer’s disease: an update on emerging drugs. Expert Opin. Emerg. Drugs 25 , 319–335 (2020).
Wang, S. et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 217 , e20200785 (2020).
Ettcheto, M. et al. Masitinib for the treatment of Alzheimer’s disease. Neurodegener. Dis. Manag. 11 , 263–276 (2021).
Maheshwari, S. et al. Navigating the dementia landscape: Biomarkers and emerging therapies. Ageing Res. Rev. 94 , 102193 (2024).
Lawlor, B. et al. Nilvadipine in mild to moderate Alzheimer disease: A randomised controlled trial. PLoS Med. 15 , e1002660 (2018).
Padhi, D. & Govindaraju, T. Mechanistic Insights for Drug Repurposing and the Design of Hybrid Drugs for Alzheimer’s Disease. J. Med. Chem. 65 , 7088–7105 (2022).
Cacabelos, R. What have we learnt from past failures in Alzheimer’s disease drug discovery? Expert Opin. Drug Discov. 17 , 309–323 (2022).
Imbimbo, B. P., Watling, M., Imbimbo, C. & Nisticò, R. Plasma ATN(I) classification and precision pharmacology in Alzheimer’s disease. Alzheimers Dement. 19 , 4729–4734 (2023).
Tatulian, S. A. Challenges and hopes for Alzheimer’s disease. Drug Discov. Today 27 , 1027–1043 (2022).
Goldman, D. P., Fillit, H. & Neumann, P. Accelerating Alzheimer’s disease drug innovations from the research pipeline to patients. Alzheimers Dement. 14 , 833–836 (2018).
Moutinho, S. The long road to a cure for Alzheimer’s disease is paved with failures. Nat. Med. 28 , 2228–2231 (2022).
Zhang, L. et al. Advance of sporadic Alzheimer’s disease animal models. Med. Res. Rev. 40 , 431–458 (2020).
Xia, Z. D. et al. Pathogenesis, Animal Models, and Drug Discovery of Alzheimer’s Disease. J. Alzheimers Dis. 94 , 1265–1301 (2023).
LaFerla, F. M. & Green, K. N. Animal models of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2 , a006320 (2012).
Chen, Z. Y. & Zhang, Y. Animal models of Alzheimer’s disease: Applications, evaluation, and perspectives. Zool. Res. 43 , 1026–1040 (2022).
Luo, Y. & Li, H. Structure-Based Inhibitor Discovery of Class I Histone Deacetylases (HDACs). Int J. Mol. Sci. 21 , 8828 (2020).
Liu, C. S. et al. Selective inhibitors of bromodomain BD1 and BD2 of BET proteins modulate radiation-induced profibrotic fibroblast responses. Int. J. Cancer 151 , 275–286 (2022).
Yu, J., Zhang, C. & Song, C. Pan- and isoform-specific inhibition of Hsp90: Design strategy and recent advances. Eur. J. Med. Chem. 238 , 114516 (2022).
Ghiboub, M. et al. Selective Targeting of Epigenetic Readers and Histone Deacetylases in Autoimmune and Inflammatory Diseases: Recent Advances and Future Perspectives. J. Pers. Med. 11 , 336 (2021).
Hu, X. L. et al. Stereoisomers of Schisandrin B Are Potent ATP Competitive GSK-3beta Inhibitors with Neuroprotective Effects against Alzheimer’s Disease: Stereochemistry and Biological Activity. ACS Chem. Neurosci. 10 , 996–1007 (2019).
Rippin, I. et al. Discovery and Design of Novel Small Molecule GSK-3 Inhibitors Targeting the Substrate Binding Site. Int J. Mol. Sci. 21 , 8709 (2020).
Beurel, E., Grieco, S. F. & Jope, R. S. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharm. Ther. 148 , 114–131 (2015).
Liang, Z. & Li, Q. X. Discovery of Selective, Substrate-Competitive, and Passive Membrane Permeable Glycogen Synthase Kinase-3beta Inhibitors: Synthesis, Biological Evaluation, and Molecular Modeling of New C-Glycosylflavones. ACS Chem. Neurosci. 9 , 1166–1183 (2018).
Silva-Garcia, O. et al. GSK3alpha: An Important Paralog in Neurodegenerative Disorders and Cancer. Biomolecules 10 , 1683 (2020).
Amaral, B. et al. Elucidation of the GSK3alpha Structure Informs the Design of Novel, Paralog-Selective Inhibitors. ACS Chem. Neurosci. 14 , 1080–1094 (2023).
Wei, J. et al. Development of inhibitors targeting glycogen synthase kinase-3β for human diseases: Strategies to improve selectivity. Eur. J. Med. Chem. 236 , 114301 (2022).
Bernard-Gauthier, V. et al. Structural Basis for Achieving GSK-3beta Inhibition with High Potency, Selectivity, and Brain Exposure for Positron Emission Tomography Imaging and Drug Discovery. J. Med. Chem. 62 , 9600–9617 (2019).
Wu, Y. W. et al. Identification of selective dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors and their effects on tau and microtubule. Int J. Biol. Macromol. 259 , 129074 (2024).
Fernandez Bessone, I. et al. DYRK1A regulates the bidirectional axonal transport of APP in human-derived neurons. J. Neurosci. 42 , 6344–6358 (2022).
Deboever, E., Fistrovich, A., Hulme, C. & Dunckley, T. The Omnipresence of DYRK1A in Human Diseases. Int J. Mol. Sci. 23 , 9355 (2022).
Miyazaki, Y. et al. Structure-activity relationship for the folding intermediate-selective inhibition of DYRK1A. Eur. J. Med. Chem. 227 , 113948 (2022).
Bieliauskas, A. V. & Pflum, M. K. Isoform-selective histone deacetylase inhibitors. Chem. Soc. Rev. 37 , 1402–1413 (2008).
Shukla, S. & Tekwani, B. L. Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation. Front. Pharm. 11 , 537 (2020).
Simões-Pires, C. et al. HDAC6 as a target for neurodegenerative diseases: what makes it different from the other HDACs? Mol. Neurodegener. 8 , 7 (2013).
Zhang, Q. Q., Zhang, W. J. & Chang, S. HDAC6 inhibition: a significant potential regulator and therapeutic option to translate into clinical practice in renal transplantation. Front. Immunol. 14 , 1168848 (2023).
Vögerl, K. et al. Synthesis and Biological Investigation of Phenothiazine-Based Benzhydroxamic Acids as Selective Histone Deacetylase 6 Inhibitors. J. Med. Chem. 62 , 1138–1166 (2019).
Li, Y. et al. Inhibition of Histone Deacetylase 6 (HDAC6) as a therapeutic strategy for Alzheimer’s disease: A review (2010-2020). Eur. J. Med. Chem. 226 , 113874 (2021).
Wang, X. X., Wan, R. Z. & Liu, Z. P. Recent advances in the discovery of potent and selective HDAC6 inhibitors. Eur. J. Med. Chem. 143 , 1406–1418 (2018).
Wang, X. X. et al. Synthesis and biological evaluation of selective histone deacetylase 6 inhibitors as multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 225 , 113821 (2021).
Liang, T. et al. Targeting histone deacetylases for cancer therapy: Trends and challenges. Acta Pharm. Sin. B 13 , 2425–2463 (2023).
He, F. et al. Melatonin- and Ferulic Acid-Based HDAC6 Selective Inhibitors Exhibit Pronounced Immunomodulatory Effects In Vitro and Neuroprotective Effects in a Pharmacological Alzheimer’s Disease Mouse Model. J. Med. Chem. 64 , 3794–3812 (2021).
Lee, H. Y. et al. 5-Aroylindoles Act as Selective Histone Deacetylase 6 Inhibitors Ameliorating Alzheimer’s Disease Phenotypes. J. Med. Chem. 61 , 7087–7102 (2018).
Cescon, E. et al. Scaffold Repurposing of in-House Chemical Library toward the Identification of New Casein Kinase 1 delta Inhibitors. ACS Med. Chem. Lett. 11 , 1168–1174 (2020).
Cho, H. & Hah, J.-M. J. B. A perspective on the development of c-jun N-terminal kinase inhibitors as therapeutics for alzheimer’s disease: Investigating structure through docking studies. Biomedicines 9 , 1431 (2021).
Kim, M. H. et al. Syntheses and biological evaluation of 1-heteroaryl-2-aryl-1H-benzimidazole derivatives as c-Jun N-terminal kinase inhibitors with neuroprotective effects. Bioorg. Med. Chem. 21 , 2271–2285 (2013).
Jang, M. et al. Discovery of 1-Pyrimidinyl-2-Aryl-4,6-Dihydropyrrolo [3,4-d]Imidazole-5(1H)-Carboxamide as a Novel JNK Inhibitor. Int J. Mol. Sci. 21 , 1698 (2020).
Jun, J. et al. Discovery of novel imidazole chemotypes as isoform-selective JNK3 inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 245 , 114894 (2023).
Guenette, R. G. et al. Target and tissue selectivity of PROTAC degraders. Chem. Soc. Rev. 51 , 5740–5756 (2022).
Sutanto, F., Konstantinidou, M. & Dömling, A. Covalent inhibitors: a rational approach to drug discovery. RSC Med. Chem. 11 , 876–884 (2020).
Cescon, E. et al. Scaffold Repurposing of in-House Chemical Library toward the Identification of New Casein Kinase 1 δ Inhibitors. ACS Med. Chem. Lett. 11 , 1168–1174 (2020).
Maramai, S., Benchekroun, M., Gabr, M. T. & Yahiaoui, S. Multitarget Therapeutic Strategies for Alzheimer’s Disease: Review on Emerging Target Combinations. Biomed. Res. Int. 2020 , 5120230 (2020).
Zou, D. et al. Latest advances in dual inhibitors of acetylcholinesterase and monoamine oxidase B against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 38 , 2270781 (2023).
Raghavendra, N. M. et al. Dual or multi-targeting inhibitors: The next generation anticancer agents. Eur. J. Med. Chem. 143 , 1277–1300 (2018).
Ferreira, J. P. S. et al. Dual-target compounds for Alzheimer’s disease: Natural and synthetic AChE and BACE-1 dual-inhibitors and their structure-activity relationship (SAR). Eur. J. Med. Chem. 221 , 113492 (2021).
Miao, S. et al. Aaptamine - a dual acetyl - and butyrylcholinesterase inhibitor as potential anti-Alzheimer’s disease agent. Pharm. Biol. 60 , 1502–1510 (2022).
Makhaeva, G. F. et al. New Hybrids of 4-Amino-2,3-polymethylene-quinoline and p-Tolylsulfonamide as Dual Inhibitors of Acetyl- and Butyrylcholinesterase and Potential Multifunctional Agents for Alzheimer’s Disease Treatment. Molecules 25 , 3915 (2020).
Bondzic, A. M. et al. Aminoalcoholate-driven tetracopper(II) cores as dual acetyl and butyrylcholinesterase inhibitors: Experimental and theoretical elucidation of mechanism of action. J. Inorg. Biochem. 205 , 110990 (2020).
Viayna, E. et al. Discovery of a Potent Dual Inhibitor of Acetylcholinesterase and Butyrylcholinesterase with Antioxidant Activity that Alleviates Alzheimer-like Pathology in Old APP/PS1 Mice. J. Med. Chem. 64 , 812–839 (2021).
He, Q. et al. Coumarin-dithiocarbamate hybrids as novel multitarget AChE and MAO-B inhibitors against Alzheimer’s disease: Design, synthesis and biological evaluation. Bioorg. Chem. 81 , 512–528 (2018).
Li, X. et al. Design, Synthesis, and Biological Evaluation of Novel Chromanone Derivatives as Multifunctional Agents for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 13 , 3488–3501 (2022).
Schneider, L. S. et al. Low-dose ladostigil for mild cognitive impairment: A phase 2 placebo-controlled clinical trial. Neurology 93 , e1474–e1484 (2019).
Stern, N. et al. Dual Inhibitors of AChE and BACE-1 for Reducing Abeta in Alzheimer’s Disease: From In Silico to In Vivo. Int. J. Mol. Sci. 23 , 13098 (2022).
Fronza, M. G., Alves, D., Praticò, D. & Savegnago, L. The neurobiology and therapeutic potential of multi-targeting β-secretase, glycogen synthase kinase 3β and acetylcholinesterase in Alzheimer’s disease. Ageing Res. Rev. 90 , 102033 (2023).
Jiang, X. Y. et al. Dual GSK-3beta/AChE Inhibitors as a New Strategy for Multitargeting Anti-Alzheimer’s Disease Drug Discovery. ACS Med. Chem. Lett. 9 , 171–176 (2018).
Jiang, X. et al. Rational design and biological evaluation of a new class of thiazolopyridyl tetrahydroacridines as cholinesterase and GSK-3 dual inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 207 , 112751 (2020).
Swetha, R. et al. Combined ligand-based and structure-based design of PDE 9A inhibitors against Alzheimer’s disease. Mol. Divers. 26 , 2877–2892 (2022).
Sheng, J. et al. Inhibition of phosphodiesterase: A novel therapeutic target for the treatment of mild cognitive impairment and Alzheimer’s disease. Front. Aging Neurosci. 14 , 1019187 (2022).
Liu, J. et al. Discovery of novel 2,3-dihydro-1H-inden-1-ones as dual PDE4/AChE inhibitors with more potency against neuroinflammation for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 238 , 114503 (2022).
Liu, Y. et al. Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy. J. Hematol. Oncol. 15 , 89 (2022).
Jakubik, J. & El-Fakahany, E. E. Current Advances in Allosteric Modulation of Muscarinic Receptors. Biomolecules 10 , 325 (2020).
Xie, X. et al. Recent advances in targeting the “undruggable” proteins: from drug discovery to clinical trials. Signal Transduct. Target Ther. 8 , 335 (2023).
Dwomoh, L., Tejeda, G. S. & Tobin, A. B. Targeting the M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neuronal. Signal 6 , NS20210004 (2022).
van der Westhuizen, E. T. et al. Fine Tuning Muscarinic Acetylcholine Receptor Signaling Through Allostery and Bias. Front. Pharm. 11 , 606656 (2020).
Luo, J. E. & Li, Y. M. Turning the tide on Alzheimer’s disease: modulation of γ-secretase. Cell Biosci. 12 , 2 (2022).
De Strooper, B. & Karran, E. New precision medicine avenues to the prevention of Alzheimer’s disease from insights into the structure and function of γ-secretases. EMBO J. 43 , 887–903 (2024).
Rynearson, K. D. et al. Preclinical validation of a potent γ-secretase modulator for Alzheimer’s disease prevention. J. Exp. Med. 218 , e20202560 (2021).
Hollingsworth, S. A. et al. Cryptic pocket formation underlies allosteric modulator selectivity at muscarinic GPCRs. Nat. Commun. 10 , 3289 (2019).
Terry, A. V. Jr., Jones, K. & Bertrand, D. Nicotinic acetylcholine receptors in neurological and psychiatric diseases. Pharm. Res. 191 , 106764 (2023).
Letsinger, A. C., Gu, Z. & Yakel, J. L. α7 nicotinic acetylcholine receptors in the hippocampal circuit: taming complexity. Trends Neurosci. 45 , 145–157 (2022).
Sinha, N. et al. Discovery of Novel, Potent, Brain-Permeable, and Orally Efficacious Positive Allosteric Modulator of α7 Nicotinic Acetylcholine Receptor [4-(5-(4-Chlorophenyl)-4-methyl-2-propionylthiophen-3-yl)benzenesulfonamide]: Structure-Activity Relationship and Preclinical Characterization. J. Med. Chem. 63 , 944–960 (2020).
Kurimoto, E. et al. An Approach to Discovering Novel Muscarinic M(1) Receptor Positive Allosteric Modulators with Potent Cognitive Improvement and Minimized Gastrointestinal Dysfunction. J. Pharm. Exp. Ther. 364 , 28–37 (2018).
Jörg, M. et al. 6-Phenylpyrimidin-4-ones as Positive Allosteric Modulators at the M(1) mAChR: The Determinants of Allosteric Activity. ACS Chem. Neurosci. 10 , 1099–1114 (2019).
Dallagnol, J. C. C. et al. Synthesis and Pharmacological Evaluation of Heterocyclic Carboxamides: Positive Allosteric Modulators of the M1 Muscarinic Acetylcholine Receptor with Weak Agonist Activity and Diverse Modulatory Profiles. J. Med. Chem. 61 , 2875–2894 (2018).
Querfurth, H. et al. A PDK-1 allosteric agonist neutralizes insulin signaling derangements and beta-amyloid toxicity in neuronal cells and in vitro. PLoS One 17 , e0261696 (2022).
Dahlstrom, M. et al. Identification of Novel Positive Allosteric Modulators of Neurotrophin Receptors for the Treatment of Cognitive Dysfunction. Cells 10 , 1871 (2021).
Önnestam, K. et al. Safety, Tolerability, Pharmacokinetics and Quantitative Electroencephalography Assessment of ACD856, a Novel Positive Allosteric Modulator of Trk-Receptors Following Multiple Doses in Healthy Subjects. J. Prev. Alzheimers Dis. 10 , 778–789 (2023).
Chen, L. et al. Advances in RIPK1 kinase inhibitors. Front. Pharm. 13 , 976435 (2022).
Geoffroy, C., Paoletti, P. & Mony, L. Positive allosteric modulation of NMDA receptors: mechanisms, physiological impact and therapeutic potential. J. Physiol. 600 , 233–259 (2022).
Balboni, B. et al. GSK-3β Allosteric Inhibition: A Dead End or a New Pharmacological Frontier? Int. J. Mol. Sci. 24 , 7541 (2023).
Christopoulos, A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov. 1 , 198–210 (2002).
Mannes, M., Martin, C., Menet, C. & Ballet, S. Wandering beyond small molecules: peptides as allosteric protein modulators. Trends Pharm. Sci. 43 , 406–423 (2022).
Coughlin, Q. et al. Allosteric Modalities for Membrane-Bound Receptors: Insights from Drug Hunting for Brain Diseases. J. Med. Chem. 62 , 5979–6002 (2019).
Zheng, L. et al. Development of covalent inhibitors: Principle, design, and application in cancer. MedComm. Oncol. 2 , e56 (2023).
Cully, M. Novel chemistry for covalent inhibitors. Nat. Rev. Drug Discov. 19 , 754 (2020).
Abdeldayem, A. et al. Advances in covalent kinase inhibitors. Chem. Soc. Rev. 49 , 2617–2687 (2020).
Jones, M. R. et al. Modulation of the Aβ peptide aggregation pathway by KP1019 limits Aβ-associated neurotoxicity. Metallomics 7 , 129–135 (2015).
Eden, A. et al. Covalent fragment inhibits intramembrane proteolysis. Front. Mol. Biosci. 9 , 958399 (2022).
Zhou, R. et al. Recognition of the amyloid precursor protein by human γ-secretase. Science 363 , eaaw0930 (2019).
Schaefer, D. & Cheng, X. Recent Advances in Covalent Drug Discovery. Pharmaceuticals 16 , 663 (2023).
McCormick, F. Sticking it to KRAS: Covalent Inhibitors Enter the Clinic. Cancer Cell 37 , 3–4 (2020).
Baillie, T. A. Targeted Covalent Inhibitors for Drug Design. Angew. Chem. Int. Ed. Engl. 55 , 13408–13421 (2016).
Bhatia, S., Singh, M., Singh, T. & Singh, V. Scrutinizing the Therapeutic Potential of PROTACs in the Management of Alzheimer’s Disease. Neurochem. Res. 48 , 13–25 (2023).
Fang, Y. et al. Progress and Challenges in Targeted Protein Degradation for Neurodegenerative Disease Therapy. J. Med. Chem. 65 , 11454–11477 (2022).
Qu, L. et al. Discovery of PT-65 as a highly potent and selective Proteolysis-targeting chimera degrader of GSK3 for treating Alzheimer’s disease. Eur. J. Med. Chem. 226 , 113889 (2021).
Sibley, C. D. & Schneekloth, J. S. Heterobifunctional molecules tackle targeted protein dephosphorylation. Trends Pharm. Sci. 43 , 263–265 (2022).
Hu, Z. et al. Targeted Dephosphorylation of Tau by Phosphorylation Targeting Chimeras (PhosTACs) as a Therapeutic Modality. J. Am. Chem. Soc . 145 , 4045–4055 (2023).
Wu, D. et al. Small molecules targeting protein-protein interactions for cancer therapy. Acta Pharm. Sin. B 13 , 4060–4088 (2023).
Kumar, V. & Roy, K. Protein-protein interaction network analysis for the identification of novel multi-target inhibitors and target miRNAs against Alzheimer’s disease. Adv. Protein Chem. Struct. Biol. 139 , 405–467 (2024).
Chen, H. et al. Network integration and protein structural binding analysis of neurodegeneration-related interactome. Brief. Bioinform. 24 , bbad237 (2023).
Ganeshpurkar, A. et al. Protein-Protein Interactions and Aggregation Inhibitors in Alzheimer’s Disease. Curr. Top. Med. Chem. 19 , 501–533 (2019).
Santini, B. L. & Zacharias, M. Rapid in silico Design of Potential Cyclic Peptide Binders Targeting Protein-Protein Interfaces. Front. Chem. 8 , 573259 (2020).
Lee, A. C., Harris, J. L., Khanna, K. K. & Hong, J. H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 20 , 2383 (2019).
Zhao, P. et al. LILRB2-mediated TREM2 signaling inhibition suppresses microglia functions. Mol. Neurodegener. 17 , 44 (2022).
Cao, Q. et al. Inhibiting amyloid-β cytotoxicity through its interaction with the cell surface receptor LilrB2 by structure-based design. Nat. Chem. 10 , 1213–1221 (2018).
Lao, K. et al. Identification of novel Aβ-LilrB2 inhibitors as potential therapeutic agents for Alzheimer’s disease. Mol. Cell Neurosci. 114 , 103630 (2021).
Ciccone, L. et al. The Positive Side of the Alzheimer’s Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1. Molecules 25 , 2439 (2020).
Cotrina, E. Y. et al. Targeting transthyretin in Alzheimer’s disease: Drug discovery of small-molecule chaperones as disease-modifying drug candidates for Alzheimer’s disease. Eur. J. Med. Chem. 226 , 113847 (2021).
Sun, Y. et al. Direct inhibition of Keap1-Nrf2 Protein-Protein interaction as a potential therapeutic strategy for Alzheimer’s disease. Bioorg. Chem. 103 , 104172 (2020).
Sun, Y. et al. A potent phosphodiester Keap1-Nrf2 protein-protein interaction inhibitor as the efficient treatment of Alzheimer’s disease. Redox Biol. 64 , 102793 (2023).
Georgakopoulos, N. et al. Phenyl Bis-Sulfonamide Keap1-Nrf2 Protein-Protein Interaction Inhibitors with an Alternative Binding Mode. J. Med. Chem. 65 , 7380–7398 (2022).
Modell, A. E., Blosser, S. L. & Arora, P. S. Systematic Targeting of Protein-Protein Interactions. Trends Pharm. Sci. 37 , 702–713 (2016).
Jungbauer, G. et al. Periodontal microorganisms and Alzheimer disease–A causative relationship? Periodontol. 2000 89 , 59–82 (2022).
Ryder, M. I. & Xenoudi, P. Alzheimer disease and the periodontal patient: New insights, connections, and therapies. Periodontol. 2000 87 , 32–42 (2021).
Ganz, T., Fainstein, N. & Ben-Hur, T. When the infectious environment meets the AD brain. Mol. Neurodegener. 17 , 53 (2022).
Komaroff, A. L. Can Infections Cause Alzheimer Disease? JAMA 324 , 239–240 (2020).
Marcocci, M. E. et al. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol . 28 , 808–820 (2020).
Wang, Y. et al. MAMDC2, a gene highly expressed in microglia in experimental models of Alzheimers Disease, positively regulates the innate antiviral response during neurotropic virus infection. J. Infect. 84 , 187–204 (2022).
Albaret, M. A. et al. HSV-1 cellular model reveals links between aggresome formation and early step of Alzheimer’s disease. Transl. Psychiatry 13 , 86 (2023).
Zhao, M. et al. Microbial infection promotes amyloid pathology in a mouse model of Alzheimer’s disease via modulating γ-secretase. Mol. Psychiatry 29 , 1491–1500 (2024).
Golzari-Sorkheh, M., Weaver, D. F. & Reed, M. A. COVID-19 as a Risk Factor for Alzheimer’s Disease. J. Alzheimers Dis. 91 , 1–23 (2023).
Wang, L. et al. Association of COVID-19 with New-Onset Alzheimer’s Disease. J. Alzheimers Dis. 89 , 411–414 (2022).
Wang, Q., Davis, P. B., Gurney, M. E. & Xu, R. COVID-19 and dementia: Analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. 17 , 1297–1306 (2021).
Zhang, H. et al. APOE interacts with ACE2 inhibiting SARS-CoV-2 cellular entry and inflammation in COVID-19 patients. Signal Transduct. Target Ther. 7 , 261 (2022).
Magusali, N. et al. A genetic link between risk for Alzheimer’s disease and severe COVID-19 outcomes via the OAS1 gene. Brain 144 , 3727–3741 (2021).
Ma, G. et al. SARS-CoV-2 Spike protein S2 subunit modulates γ-secretase and enhances amyloid-β production in COVID-19 neuropathy. Cell Discov. 8 , 99 (2022).
Liu, S. et al. Highly efficient intercellular spreading of protein misfolding mediated by viral ligand-receptor interactions. Nat. Commun. 12 , 5739 (2021).
Mansour, H. M. The interference between SARS-COV-2 and Alzheimer’s disease: Potential immunological and neurobiological crosstalk from a kinase perspective reveals a delayed pandemic. Ageing Res. Rev. 94 , 102195 (2024).
Wang, Y. et al. Identification of novel diagnostic panel for mild cognitive impairment and Alzheimer’s disease: findings based on urine proteomics and machine learning. Alzheimers Res. Ther. 15 , 191 (2023).
Ashton, N. J., Ide, M., Zetterberg, H. & Blennow, K. Salivary biomarkers for Alzheimer’s disease and related disorders. Neurol. Ther. 8 , 83–94 (2019).
Alber, J. et al. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimers Dement. 16 , 229–243 (2020).
Pun, F. W., Ozerov, I. V. & Zhavoronkov, A. AI-powered therapeutic target discovery. Trends Pharm. Sci. 44 , 561–572 (2023).
Cheng, F. et al. Artificial intelligence and open science in discovery of disease-modifying medicines for Alzheimer’s disease. Cell Rep. Med. 5 , 101379 (2024).
Xu, J. et al. Interpretable deep learning translation of GWAS and multi-omics findings to identify pathobiology and drug repurposing in Alzheimer’s disease. Cell Rep. 41 , 111717 (2022).
Geng, C., Wang, Z. & Tang, Y. Machine learning in Alzheimer’s disease drug discovery and target identification. Ageing Res. Rev. 93 , 102172 (2024).
Winchester, L. M. et al. Artificial intelligence for biomarker discovery in Alzheimer’s disease and dementia. Alzheimers Dement. 19 , 5860–5871 (2023).
Qiu, S. et al. Multimodal deep learning for Alzheimer’s disease dementia assessment. Nat. Commun. 13 , 3404 (2022).
Francis, P. T., Palmer, A. M., Snape, M. & Wilcock, G. K. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry 66 , 137–147 (1999).
Whitehouse, P. J. et al. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10 , 122–126 (1981).
Lawlor, B. A. & Davis, K. L. Does modulation of glutamatergic function represent a viable therapeutic strategy in Alzheimer’s disease? Biol. Psychiatry 31 , 337–350 (1992).
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharm. Sci. 12 , 383–388 (1991).
Regland, B. & Gottfries, C. G. The role of amyloid beta-protein in Alzheimer’s disease. Lancet 340 , 467–469 (1992).
Goedert, M. Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci. 16 , 460–465 (1993).
Mandelkow, E. M. & Mandelkow, E. Tau in Alzheimer’s disease. Trends Cell Biol. 8 , 425–427 (1998).
Aisen, P. S. & Davis, K. L. Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am. J. Psychiatry 151 , 1105–1113 (1994).
Heppner, F. L., Ransohoff, R. M. & Becher, B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16 , 358–372 (2015).
Chen, C. et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 71 , 2233–2252 (2022).
Chandra, S., Sisodia, S. S. & Vassar, R. J. The gut microbiome in Alzheimer’s disease: what we know and what remains to be explored. Mol. Neurodegener. 18 , 9 (2023).
Bush, A. I. et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265 , 1464–1467 (1994).
Zatta, P., Drago, D., Bolognin, S. & Sensi, S. L. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharm. Sci. 30 , 346–355, (2009).
Tzioras, M., McGeachan, R. I., Durrant, C. S. & Spires-Jones, T. L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 19 , 19–38 (2023).
Zhang, Y. et al. Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct. Target Ther. 8 , 248 (2023).
Kaur, D., Sharma, V. & Deshmukh, R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 27 , 663–677 (2019).
Stanca, S., Rossetti, M. & Bongioanni, P. Astrocytes as Neuroimmunocytes in Alzheimer’s Disease: A Biochemical Tool in the Neuron-Glia Crosstalk along the Pathogenetic Pathways. Int J. Mol. Sci. 24 , 13880 (2023).
Arranz, A. M. & De Strooper, B. The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol. 18 , 406–414 (2019).
Sarkar, S. & Biswas, S. C. Astrocyte subtype-specific approach to Alzheimer’s disease treatment. Neurochem Int. 145 , 104956 (2021).
Colonna, M. The biology of TREM receptors. Nat. Rev. Immunol. 23 , 580–594 (2023).
Qin, Q. et al. TREM2, microglia, and Alzheimer’s disease. Mech. Ageing Dev. 195 , 111438 (2021).
Ulland, T. K. & Colonna, M. TREM2 - a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 14 , 667–675 (2018).
Song, W. M. et al. Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J. Exp. Med. 215 , 745–760 (2018).
Lessard, C. B. et al. High-affinity interactions and signal transduction between Aβ oligomers and TREM2. EMBO Mol. Med. 10 , e9027 (2018).
Wang, S. et al. TREM2 drives microglia response to amyloid-β via SYK-dependent and -independent pathways. Cell 185 , 4153–4169.e4119 (2022).
Custodia, A. et al. Endothelial Progenitor Cells and Vascular Alterations in Alzheimer’s Disease. Front Aging Neurosci. 13 , 811210 (2021).
Download references
Acknowledgements
This work was supported by the Supported by Sichuan Science and Technology Program (2023YFS0047, 2022NSFSC1365).
Author information
These authors contributed equally: Jifa Zhang, Yinglu Zhang, Jiaxing Wang
Authors and Affiliations
Department of Neurology, Laboratory of Neuro-system and Multimorbidity and State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, 610041, Sichuan, China
Jifa Zhang, Yinglu Zhang, Yilin Xia, Jiaxian Zhang & Lei Chen
Department of Pharmaceutical Sciences, College of Pharmacy, University of Tennessee Health Science Center, Memphis, 38163, TN, USA
Jiaxing Wang
You can also search for this author in PubMed Google Scholar
Contributions
L.C. conceived and designed this project. JF.Z., YL.Z., and JX.W. wrote the draft of the manuscript. JF.Z., YL.Z., JX.W., YL.X., and JX.Z. did the literature search and review. L.C., JF.Z., YL.Z., and JX.W. revised the manuscript. JF.Z. and YL.Z. prepared and edited the tables and figures. L.C. and JF.Z. supervised the project. All authors have read and approved the article.
Corresponding author
Correspondence to Lei Chen .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhang, J., Zhang, Y., Wang, J. et al. Recent advances in Alzheimer’s disease: mechanisms, clinical trials and new drug development strategies. Sig Transduct Target Ther 9 , 211 (2024). https://doi.org/10.1038/s41392-024-01911-3
Download citation
Received : 09 November 2023
Revised : 18 March 2024
Accepted : 02 July 2024
Published : 23 August 2024
DOI : https://doi.org/10.1038/s41392-024-01911-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Correction: the jak-stat pathway: from structural biology to cytokine engineering.
- Faming Wang
Signal Transduction and Targeted Therapy (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
COMMENTS
Alzheimer's disease articles from across Nature Portfolio. Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by ...
Alzheimer’s disease (AD) is a disorder that causes degeneration of the cells in the brain and it is the main cause of dementia, which is characterized by a decline in thinking and independence in personal daily activities. AD is considered a multifactorial disease: two main hypotheses were proposed as a cause for AD, cholinergic and amyloid ...
The Alzheimer’s community has seen remarkable progress in the past year, with the first disease-modifying therapy, lecanemab, receiving traditional approval from the U.S. Food and Drug Administration (FDA) for the treatment of early Alzheimer’s in July 2023, followed by donanemab receiving traditional approval in July 2024.
ADRCs are required to collect an annual Uniform Data Set (UDS) of clinical and neuropathological research data on clinical research participants. This wealth of combined data has supported significant advances in our understanding of Alzheimer’s disease and other neurodegenerative diseases. To date, the NACC has assembled longitudinal data ...
Dementia has emerged as a global health challenge. According to the World Health Organization’s 2022 blueprint for dementia research, an estimated 55.2 million individuals globally are affected.
The federal government’s Alzheimer’s and related dementias research strategy focuses on engaging a cross-disciplinary team of geneticists, epidemiologists, gerontologists, behavioral scientists, disease and structural biologists, pharmacologists, clinical researchers, and others to bring the greatest and most diverse expertise to the field.