- Share full article
Advertisement
Supported by

Untangling Rosalind Franklin’s Role in DNA Discovery, 70 Years On
Historians have long debated the role that Dr. Franklin played in identifying the double helix. A new opinion essay argues that she was an “equal contributor.”

By Emily Anthes
On April 25, 1953, James Watson and Francis Crick published a landmark paper in Nature, proposing the double helix as the long elusive structure of DNA, a discovery that a decade later earned the men the Nobel Prize in Physiology or Medicine.
In the final paragraph of the paper, they acknowledged that they had been “stimulated by a knowledge of the general nature of the unpublished experimental results and ideas” of two scientists at King’s College London, Maurice Wilkins and Rosalind Franklin.
In the 70 years since, a less flattering story has emerged, thanks in large part to Dr. Watson’s own best-selling book, “The Double Helix.” In the book, he not only wrote disparagingly of Dr. Franklin, whom he called Rosy, but also said that he and Dr. Crick had used her data without her knowledge.
“Rosy, of course, did not directly give us her data,” Dr. Watson wrote. “For that matter, no one at King’s realized they were in our hands.”
This account became a parable of poor scientific behavior, leading to a backlash against Dr. Watson and Dr. Crick and turning Dr. Franklin into a feminist icon. It also set off a long-running debate among historians: Precisely what role did Dr. Franklin play in the discovery of the double helix, and to what extent was she wronged?
In a new opinion essay , published in Nature on Tuesday, two scholars argue that what transpired “was less malicious than is widely assumed.” The scholars, Matthew Cobb, a zoologist and historian at the University of Manchester who is writing a biography of Dr. Crick, and Nathaniel Comfort, a historian of medicine at Johns Hopkins University who is writing a biography of Dr. Watson, draw upon two previously overlooked documents in Dr. Franklin’s archive.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Daily Snapshot
Spread the word
DNA at 70: Untangling Rosalind Franklin’s Role
Historians have long debated the role that dr. franklin played in identifying the double helix. a new opinion essay argues that she was an “equal contributor.”.

On April 25, 1953, James Watson and Francis Crick published a landmark paper in Nature, proposing the double helix as the long elusive structure of DNA, a discovery that a decade later earned the men the Nobel Prize in Physiology or Medicine.
In the final paragraph of the paper, they acknowledged that they had been “stimulated by a knowledge of the general nature of the unpublished experimental results and ideas” of two scientists at King’s College London, Maurice Wilkins and Rosalind Franklin.
In the 70 years since, a less flattering story has emerged, thanks in large part to Dr. Watson’s own best-selling book, “The Double Helix.” In the book, he not only wrote disparagingly of Dr. Franklin, whom he called Rosy, but also said that he and Dr. Crick had used her data without her knowledge.
“Rosy, of course, did not directly give us her data,” Dr. Watson wrote. “For that matter, no one at King’s realized they were in our hands.”
This account became a parable of poor scientific behavior, leading to a backlash against Dr. Watson and Dr. Crick and turning Dr. Franklin into a feminist icon. It also set off a long-running debate among historians: Precisely what role did Dr. Franklin play in the discovery of the double helix, and to what extent was she wronged?
In a new opinion essay , published in Nature on Tuesday, two scholars argue that what transpired “was less malicious than is widely assumed.” The scholars, Matthew Cobb, a zoologist and historian at the University of Manchester who is writing a biography of Dr. Crick, and Nathaniel Comfort, a historian of medicine at Johns Hopkins University who is writing a biography of Dr. Watson, draw upon two previously overlooked documents in Dr. Franklin’s archive.
These documents, they say, suggest that Dr. Franklin knew that Dr. Watson and Dr. Crick had access to her data and that she and Dr. Wilkins collaborated with them. “We should be thinking of Rosalind Franklin, not as the victim of DNA, but as an equal contributor and collaborator to the structure,” Dr. Comfort said.
Other experts said that the new documents were interesting but did not radically change the narrative; it has long been clear that Dr. Franklin played a key role in the discovery. “What this does is add a little new evidence to a trail, which leads directly to Franklin’s being a major participant,” said David Oshinsky, a historian of medicine at New York University.
And regardless of what Dr. Franklin knew about who had access to her data, the new documents do not change the fact that she did not receive adequate recognition for her work, some historians said.
If you like this article, please sign up for Snapshot, Portside's daily summary.
(One summary e-mail a day, you can change anytime , and Portside is always free.)
“What is unequal and has always been unequal and is still unequal about Rosalind Franklin is the credit that she didn’t get in the aftermath of the discovery,” said Dr. Jacalyn Duffin, a hematologist and historian of medicine at Queen’s University, in Canada.
Seeing double

A crystallographic X-ray image from Dr. Franklin’s lab that helped identify the structure of DNA. Science History Images, via Alamy
In the early 1950s, Dr. Watson and Dr. Crick were working together at the University of Cambridge, in Britain, trying to piece together the structure of DNA, largely by building models of the molecule.
At nearby Kings College London, Dr. Franklin and Dr. Wilkins were trying to solve the same puzzle experimentally, using X-rays to create images of DNA. (They had a famously fractious relationship, and largely worked separately.)
In “The Double Helix,” Dr. Watson suggested that his breakthrough came after Dr. Wilkins showed him one of Dr. Franklin’s images, known as Photograph 51. “The instant I saw the picture my mouth fell open and my pulse began to race,” Dr. Watson wrote.
That book was published in 1968, a decade after Dr. Franklin died of ovarian cancer at age 37, and it became the prevailing narrative of the discovery. But the real story was more complex.
In December 1952, Dr. Crick’s supervisor, the molecular biologist Max Perutz, received a report on Dr. Franklin’s unpublished results during an official visit to King’s College. Dr. Perutz later gave this report to Dr. Crick and Dr. Watson.
This data proved more useful to the pair than Photograph 51, said Dr. Cobb and Dr. Comfort, who found a letter that implies Dr. Franklin knew her results had made their way to Cambridge.
In the letter, which was written in January 1953, Pauline Cowan, a scientist at King’s College, invited Dr. Crick to an upcoming talk by Dr. Franklin and her student. But, Dr. Cowan wrote, Dr. Franklin and her student said that Dr. Perutz “already knows more about it than they are likely to get across so you may not think it worthwhile coming.”
That letter “strongly suggests” that Dr. Franklin knew the Cambridge researchers had access to her data and that she “doesn’t seem to have minded,” Dr. Cobb said.
Dr. Cobb and Dr. Comfort also found a draft of a never-published Time magazine article about the discovery of the double helix. The draft characterized the research not as a race but as the product of two teams that were working in parallel and occasionally conferring with each other.
“It portrays the work on the double helix, the solving of the double helix, as the work of four equal contributors,” Dr. Comfort said.
A question of credit

Historians say there is no evidence of ill will from Dr. Franklin, who became friendly with Dr. Watson and Dr. Crick in the final years of her brief life. Science History Images, via Alamy
Elspeth Garman, a molecular biophysicist at the University of Oxford, said that she agreed with Dr. Comfort and Dr. Cobb’s conclusion, saying, “They got right that she was a full participant.”
But Dr. Perutz’s sharing of Dr. Franklin’s unpublished data is “slightly iffy,” she said. (In 1969, Dr. Perutz wrote that the report was not confidential but that he should have asked for permission to share it “as a matter of courtesy.”)
Still, other scientists and historians said they were puzzled by the arguments made in the Nature essay. Helen Berman, a structural biologist at Rutgers University, called them “sort of strange.” Of Dr. Franklin, she said, “If she was an equal member, then I don’t know that she was treated very well.”
Dr. Franklin and Dr. Wilkins each published their own results in the same issue of Nature that included Dr. Watson and Dr. Crick’s report, as part of a package of papers. But Dr. Berman wondered why the scientists did not collaborate on a single paper with shared authorship. And several scholars said that they thought the new essay minimized the wrongdoing by the Cambridge team.
Dr. Comfort said that he and Dr. Cobb were not “trying to exonerate” Dr. Watson and Dr. Crick, whom he said were “slow to fully acknowledge” Dr. Franklin’s contribution. Dr. Cobb said that the Cambridge scientists should have told Dr. Franklin that they were using her data. “They were ungallant,” he said. “They were not as open as they should have been.” But, he added, it wasn’t “theft.”
There is no evidence that Dr. Franklin felt aggrieved by what happened, historians said, and she became friendly with the Cambridge duo in the final years of her brief life. “As far as I can tell, there was no bad feeling,” Dr. Oshinsky said.
That might have changed had Dr. Franklin lived long enough to read “The Double Helix,” several scholars noted. “‘The Double Helix’ is just appalling,” Dr. Garman said. “It gives a very, very slanted view, and doesn’t give her the credit for the bits that they even used from her.”
Dr. Franklin’s early death also meant she missed out on the Nobel Prize, but the Nobel Assembly could have found other ways to acknowledge her contribution, said Nils Hansson, a historian of medicine at Heinrich Heine University Düsseldorf, in Germany. Neither Dr. Watson nor Dr. Crick mentioned her when they accepted their awards, Dr. Hansson noted, although Dr. Wilkins, who also received the prize, did.
“She truly did get a raw deal,” said Dr. Howard Markel, a physician and historian of medicine at the University of Michigan and the author of “The Secret of Life,” a book about the discovery of the double helix. “Everyone likes to receive proper credit for their work. Everyone should care enough about their colleagues to ensure the process of fair play.”
Emily Anthes is a reporter for The Times, where she focuses on science and health and covers topics like the coronavirus pandemic, vaccinations, virus testing and Covid in children. More about Emily Anthes
A version of this article appears in print on May 2, 2023, New York Times with the headline: Essay Extends Debate Over DNA Discovery. Subscribe
- Rosalind Franklin
- Women in Science
- History Classics
- Your Profile
- Find History on Facebook (Opens in a new window)
- Find History on Twitter (Opens in a new window)
- Find History on YouTube (Opens in a new window)
- Find History on Instagram (Opens in a new window)
- Find History on TikTok (Opens in a new window)
- This Day In History
- History Podcasts
- History Vault
Rosalind Franklin’s Overlooked Role in the Discovery of DNA’s Structure
By: Sarah Pruitt
Published: March 25, 2024

It’s one of the most famous moments in the history of science: On February 28, 1953, Cambridge University molecular biologists James Watson and Francis Crick determined that the structure of deoxyribonucleic acid, or DNA—the molecule carrying the genetic code unique to any individual—was a double helix polymer, a spiral consisting of two strands of DNA wound around one another.
Nearly 10 years later, Watson and Crick, along with biophysicist Maurice Wilkins, received the 1962 Nobel Prize in Physiology or Medicine for uncovering what they called the “secret of life.” Yet another person was missing from the award ceremony, whose work was vital to the discovery of DNA’s structure. Rosalind Franklin was a chemist and X-ray crystallographer who studied DNA at King’s College London from 1951 to 1953, and her unpublished data paved the way for Watson and Crick’s breakthrough.
An Unflattering Portrayal in Watson's Account
Franklin, who died of ovarian cancer in 1958 at the age of 37, was ineligible to receive the Nobel, which is not given posthumously. Yet debate over her role in the discovery of DNA’s structure and her failure to be recognized for it began simmering after the publication of Watson’s bestselling book The Double Helix: A Personal Account of the Discovery of the Structure of DNA in 1968 and its highly unflattering portrait of Franklin.
“Watson portrayed Franklin as this kind of evil figure—a schoolmarmish, shrewish person,” says Nathaniel Comfort, a historian of medicine at Johns Hopkins University who is working on a biography of the famed molecular biologist. Watson also related in his book that he and Crick had gained access to Franklin’s data without her knowledge, including the now-famous Photograph 51, an X-ray image of DNA that immediately convinced Watson that the molecule’s structure must be a helix.
Watson’s treatment of Franklin in The Double Helix provoked a robust backlash among those who viewed her as a victim of betrayal, sexism and misogyny, including Franklin’s friend Anne Sayre, who published a biography of Franklin in 1975 . Comfort argues that this view also obscures the more complicated truth of Franklin’s contributions. As he and Matthew Cobb argued in a 2023 article in Nature , a reconsideration of the available evidence suggests that Franklin should be recognized not as a martyr, but as an equal contributor to solving the double helix structure of DNA.
Rosalind Franklin: Expert Crystallographer

In 1951, Franklin joined a team of biophysicists led by John Randall at King’s College who were using X-ray crystallography to study DNA. The molecule had been discovered in 1869, but its structure and function weren’t yet understood. After learning X-ray crystallography at a government-run lab in France, she was already an expert in the scientific technique, which involves beaming X-rays at crystalline structures and taking photographs of the patterns created by atoms in the structures diffracting the X-rays. By measuring the sizes, angles and intensities of the patterns, researchers can create a 3-D picture of the crystalline structure.
From the beginning, Franklin famously clashed with Wilkins, who was Randall's deputy, and the two began working largely separately from one another. Wilkins had previously identified two forms of DNA appearing in the X-ray images; Franklin discovered that by adjusting the level of humidity in the specimen chamber, she could convert the crystalline, relatively dry “A” form of DNA into the wetter, paracrystalline “B” form. She shared these key insights into DNA at a seminar in November 1951, which Watson attended.
“Her notes for that lecture are very detailed,” Comfort says, adding that Franklin initially assumed both the A and B forms had a helical structure. “She describes DNA as a big helix, describes the two forms and lays out their differences…and [explains] how the structure switches from A to B depending on the relative humidity in the sample chamber.”
Franklin’s ‘Photograph 51’
Despite capturing clear evidence of the B form’s double helical structure—most notably in what became known as Photograph 51, taken in May 1952—Franklin chose to focus on the drier A form of DNA, which produced a much sharper, more detailed image than the B form. This focus pointed her away from the idea of a helix, because the A form did not appear to be helical.
“For a chemist and an X-ray crystallographer, she was doing the [form] that made the most sense,” Comfort says. “She wasn't a biologist, and so she didn't appreciate that in a living cell, the more hydrated B form was going to be much more present, because a cell is a very wet place.”
In February 1953, Wilkins showed Photograph 51 of the B form of DNA to his friend Watson at Cambridge, who along with Crick was attempting to determine the molecule’s structure mainly through building and analyzing physical models. Wilkins received the image from Raymond Gosling, who worked for both Wilkins and Franklin and had taken the photo with Franklin.
Watson later claimed that seeing Photograph 51 immediately convinced him that a DNA helix must exist. “The instant I saw the picture my mouth fell open and my pulse began to race,” he wrote in The Double Helix . Soon after that, Crick’s supervisor passed along a report on Franklin’s unpublished results, which he had received during a visit to the King’s College lab in December 1952. By late February 1953, Watson and Crick had constructed their model of the DNA double helix, which they formally announced in a landmark paper in Nature that April.
To Comfort, Watson’s version of events doesn’t ring entirely true when it comes to Photograph 51 and its importance. “Watson talks [in The Double Helix ] about realizing only then that there was an A and a B form…but Franklin talked about that at the end of 1951, and she and Wilkins talked about it openly,” Comfort says. “I think he was writing it as though the photograph was the magic key because it made a good discovery narrative that allowed him to boil down and communicate an enormously complex, highly technical kind of science.”
Franklin’s Understanding of DNA’s Structure
Comfort also discounts the idea that Franklin, an expert crystallographer, did not understand the significance of the X-ray diffraction image she and Gosling had taken of DNA’s B form 10 months earlier. “She was way too good for that,” he says.
In fact, Franklin was simply more focused on the A form of DNA at the time, and was also in the process of leaving King’s College behind for a new job at Birkbeck College, also in London. Before she left, however, Franklin started a new laboratory notebook, with notes on the B form of DNA.
By late February 1953, Franklin’s notes reveal that she had not only accepted that DNA had a helical structure, probably with two strands; she had also recognized that the component nucleotides, or bases, on each strand were related in a way that made the strands complementary, allowing the molecule to easily replicate. “Franklin’s colleague Aaron Klug analyzed her research notes and said that Franklin was ‘two steps away’ from the double helix,” Comfort says. “Given a couple more months, she surely would have had it.”
Both Wilkins and Franklin (with Gosling) published separate papers in the same April 1953 issue of Nature , largely supporting Watson and Crick’s model of DNA’s structure. The earliest presentation of the double helix that June was signed by authors of all three papers, suggesting—as Comfort and Cobb point out in their article—that the discovery of DNA was seen at the time as a joint effort, not just the triumph of Watson and Crick.
Taking Full Measure of Franklin’s Contributions
Over the next five years, Franklin led a team of researchers studying ribonucleic acid, or RNA, in viruses such as polio and the tobacco mosaic virus (TMV). Diagnosed with ovarian cancer in 1956, Franklin continued her work until days before her death in April 1958. Franklin also remained in regular contact with Watson and Crick after she left King’s College, even becoming good friends with Crick and his wife, Odile.
Franklin’s unjust exclusion from the Nobel Prize, combined with Watson’s decidedly sexist portrayal in The Double Helix led many to see her as a victim of chauvinism and betrayal. A more complicated view of events reveals a scientist who was an equal contributor to the discovery of DNA’s structure, as well as a trailblazer in the all-important field of virology.
“Franklin had an incredible series of insights into how the RNA is packed within the protein shell of TMV,” Comfort says. “She was widely recognized and seen as being at the top of her field.”

HISTORY Vault: Women's History
Stream acclaimed women's history documentaries in HISTORY Vault.

Sign up for Inside History
Get HISTORY’s most fascinating stories delivered to your inbox three times a week.
By submitting your information, you agree to receive emails from HISTORY and A+E Networks. You can opt out at any time. You must be 16 years or older and a resident of the United States.
More details : Privacy Notice | Terms of Use | Contact Us

Realizing the benefits of human genetics and genomics research for people everywhere.
Annual DNA Day Essay Contest

ASHG is proud to support National DNA Day through the Annual DNA Day Essay Contest. DNA Day commemorates the completion of the Human Genome Project in April 2003 and the discovery of the double helix of DNA in 1953.
This contest is open to students in grades 9-12 worldwide and asks students to examine, question, and reflect on important concepts in genetics. Essays are expected to be well-reasoned arguments that indicate a deep understanding of scientific concepts related to the essay question. They are evaluated by ASHG members through three rounds of scoring.
2024 Question
Many human diseases have a genetic component. Some diseases result from a change in a single gene or even multiple genes. Yet, many diseases are complex and stem from an interaction between genes and the environment. Environmental factors may include chemicals in the air or water, nutrition, microbes, ultraviolet radiation from the sun and social context. Provide an example of how the interplay of genetics and environment can shape human health.
Important Dates
- Early January, 2024: Submission site opens
- March 6, 2024: Submission site closes
- April 25, 2024: DNA Day! Winners and Honorable Mentions announced
1st Place Winner: $1,000 for student $1,000 genetics materials grant
2nd Place Winner: $600 for student $600 genetics materials grant
3rd Place Winner: $400 for student $400 genetics materials grant
Honorable Mentions : 10 student prizes of $100 each
Questions? Email [email protected]
The rubric below is used by judges to evaluate every essay in the second and third rounds of judging.
| Overall accuracy of the science content | 0-6 |
| Use of evidence in support of an argument/answer; essay well-focused on the question/topic selected | 0-6 |
| Writing quality (clear thesis, composition, grammar, syntax, spelling) | 0-5 |
| References and citations (quality and appropriateness) | 0-3 |
|
|
Rules & Requirements
- No LLM (large-language model) tool will be accepted as a credited author on this essay. That is because any attribution of authorship carries with it accountability for the work, and AI tools cannot take such responsibility. Students using LLM tools should document this use in the citations section.
- Essays must be submitted by a teacher or administrator and written by high school students (grades 9-12) in the U.S. and internationally. Parents may submit essays if the student is home schooled.
- Essays must be written by one individual student; group submissions are not permitted.
- Essays must be in English and no more than 750 words. Word count includes in-text citations, but not reference lists.
- Submissions should not include the student’s name in the essay text. This helps with impartial judging.
- Essays must include at least one reference. References should be clearly documented with both in-text citations and in the references list. The reference list should be separately entered in the “References” section of the submission page.
- APA or MLA style can be used for citations. There is no limit on how many references students may use, but they should avoid too many references, as judges want to know the student’s opinion on the question and not the opinion of the resources.
- Quality of references will be considered by judges when scoring.
- Only classroom teachers are eligible for the equipment grant.
- Teachers of first-place winners from 2020, 2021, 2022, and 2023 are not eligible for equipment grants in 2024.
Please Note Text from essays may be used for research purposes to identify misconceptions, misunderstandings, and areas of student interest in genetics. Student text may be published on the ASHG website, newsletter, or in other ASHG publications.
Plagiarism will not be tolerated. The text of the student’s essay must be his or her own words unless quotations are explicitly noted. If plagiarism is suspected during any point of the contest, the essay in question will be examined. Essays found to contain the uncited work of others will be disqualified and the student’s teacher will be notified. Plagiarism.org gives a helpful explanation of what plagiarism is.
How many essays can one student submit? Only one entry per student.
How many essays can one teacher submit on behalf of students? Each teacher may submit up to six student essays per class, for up to three classes.
What are low-quality a high-quality sources? A low-quality source is one that doesn’t guarantee accurate information, such as Wikipedia. High-quality sources include research journals, such as those accessible through PubMed.
What is included in the 750-word count, and what is not?
- All text in the essay, in-line citations/references, headings and titles, and image captions are included in the word count
- The reference list is the only text not included in the word count.
Should references have a separate page? The reference list will be submitted separately in the “references” section of the submission site. Everything will be included on one page once the essay is submitted.
Is there a standard font or margin size preferred? No. Once the essay is copied and pasted into the submission site, it will be formatted to fit our standard margins and fonts.
How do I submit my essay if my teacher cannot do it for me? Try to find any other teacher or guidance counselor at your school who can submit for you. If this isn’t an option, please email us at [email protected] .
Can my guidance counselor or another school administrator submit my essay for me? Yes.
Can I submit for my student who is currently studying abroad? Students must be studying at the same school as the teacher who submits their essays.
Can I change information after I have submitted? No, please make sure all information is correct before submitting because it will be final.
How does the teacher vouch for the originality of the student’s work? Your submission represents your authentication that the essays are the original work of your students.
I submitted late. Will my essay still be judged? Late submissions will not be judged.
Where’s the confirmation email? It may take some time for the email to get to you. If you haven’t received it by the end of the day, either check your junk mailbox or double check that the email address you provided is correct. If neither of those options work, email [email protected] .
Summarized below are some of the most common issues judges note in reading submitted essays.
- Too much focus on details. A focus on details to the detriment of demonstrating a clear understanding of the big picture. Judges are much more forgiving of errors in details than errors in fundamental concepts and larger ideas.
- Overstating. Sweeping and grandiose overstatements of the current/future state and/or utility of biotechnology or biomedical science.
- Inaccuracy in technical language. Judges know you do not know all the “science jargon,” so don’t feel obligated to use it.
- Lack of in-text citations in, or lack of citations for information that is not considered common knowledge. If you got the information from somewhere else, cite the source.
- Using out-of-date references. Scientific understanding changes very rapidly, and references that are more than five years old are likely to have outdated ideas.
- Using too many quotes. Although occasional use is warranted, too many quotes lead judges to think the author doesn’t grasp the topic.
Check out the links below for excerpts from past winners’ essays!
Want to become a judge? If you are a current-year ASHG member, you will receive an email each February inviting you to volunteer. If you did not receive the email or cannot locate it, please contact [email protected] . You can also volunteer by the visiting the ASHG involvement page. You may forward the judge recruiting email ONLY to fellow ASHG current members. The deadline to sign up as a judge is the usually the end of February for that year’s Contest. If you have questions about future years, please contact [email protected]
Follow @DNAday
ASHG uses cookies to provide you with a secure and custom web experience. Privacy Policy
The future of DNA is unfolding now
Advances in DNA technology bring up fascinating questions about what role it will play in our society, from medicine to food

An arrest in the decades-old Golden State Killer case.
A Chinese scientist creating the first gene-edited twin baby girls.
DNA is clearly changing our reality.
In recognition of National DNA Day on April 25, scientists at Arizona State University took time to reflect on some big questions: What brought us to this point, where are we going from here — and just because we can, should we?
As is the case with most dense subjects, the best place to start is usually the beginning.
Where it all began
The average science novice might point to the Human Genome Project that had roots in the 1980s as the origin of modern DNA science. But it goes back further than that, to the discovery of the double helical structure in the 1950s and the development of the sequencing process in the 1970s that unlocked the genetic information contained in DNA.
“Those were crucial technological breakthroughs that enabled the whole field to unfold,” said Robert Cook-Deegan , professor in the School for the Future of Innovation in Society.
He witnessed firsthand as genomics took on its current form in the late 1980s, when molecular biologist James Watson — the very man who in 1953 had co-authored the paper proposing the double helix structure of the DNA molecule — asked him to lend his science and health policy expertise to the Human Genome Project.
At the time, computing technology began advancing at a rapid clip, allowing scientists to study the whole genome at once instead of one gene at a time — for the first time, they had a 30,000-foot view of the building blocks of life.
The term genomics was coined with the launch of the eponymous peer-reviewed journal in 1987 and helped to distinguish the science from genetics, the study of inheritance that only considered one gene at a time.
This newfound perspective of the curious interactions and fascinating entanglements of the chromosomes and proteins that make us who we are ushered in an era of more precise diagnostics. By analyzing a person’s genome and comparing it to relatives, scientists could pinpoint differences and similarities in their genetic makeup that might make them more prone to certain diseases or conditions.

"We’re all mountains, but we have some differences." — School of Life Sciences Assistant Professor Melissa Wilson
School of Life Sciences Assistant Professor Melissa Wilson studies the evolution of sex chromosomes and how they could be related to disease risk. In an unprecedented upcoming paper, she and a team of researchers theorize that women’s propensity toward overactive immune systems helps them both surveil and fight off cancer better than men.
She explains the utility of the human genome reference thusly:
“It’s like if I gave you a puzzle of Camelback Mountain and I said, ‘This is the human genome, it's Camelback Mountain.’ But really, some of us look like the Appalachians, and some of us look like the Superstitions, and some of us look like Four Peaks. We’re all mountains, but we have some differences. So we use that puzzle of Camelback Mountain as our reference to see where they are the same and where they are different.”
Then, in the mid-2000s, new forms of faster DNA sequencing allowed for the detection of variants in individuals and populations.

Robert Cook-Deegan
“That’s one thing nobody saw coming,” Cook-Deegan said. The ability to identify genetic differences among populations has vast implications for tracing ancestry, including the study of ancient DNA. It gave researchers insight into regional ancestry, migration patterns and more.
Nowadays, while scientists have already harnessed the potential of the naturally occurring genome editing system known as CRISPR-Cas9 to genetically modify babies in the womb, Cook-Deegan cautions we still have much more to learn.
“We’re at the toddler stage,” he said. “There’s just so much data coming out and we know so little about so much. Understanding the genome is not just about what genes you have, but understanding why and how and when they’re turned on and turned off. ... We still don’t understand that regulatory switch-work at all. We’re just at the very beginning of being able to understand that. That’s going to go on for about another century.”
The genome guides precision medicine
From the 18th through the 20th centuries, a physician's dominant tool was the microscope. They would look at cells or tissues under a microscope and then say, “This patient has disease X, Y or Z,” based on the way the cells appeared. It was very good, and took health care a long way.
Then the Human Genome Project launched. The world's largest collaborative biological project, it was an international scientific research project with the goal of determining the sequence of human DNA and identifying and mapping all of the genes of the human genome from a physical and a functional standpoint. It was completed in 2003.
“What we learned in the 21st century, or even at the very tail end of the 20th century, is that we can get even more precise about what a patient has by looking at the molecules,” said Joshua LaBaer , executive director of ASU’s Biodesign Institute and a professor in the School of Molecular Sciences. LaBaer Center director, Biodesign Virginia G. Piper Center for Personalized Diagnostics; interim center director, ASU-Banner Neurodegenerative Disease Research Center; faculty member, Biodesign Virginia G. Piper Center for Personalized Diagnostics. is one of the nation’s foremost investigators in the rapidly expanding field of personalized diagnostics.
“Precision medicine is basically a way of fine-tuning the way we treat our patients,” LaBaer said. “With personalized medicine, doctors like myself always felt we personalized treatment. We don’t treat a population; we treat an individual.”
When LaBaer went to medical school back in the 20th century, one would look at certain cells and tissues in the breast under the microscope and say “infiltrating ductal carcinoma of the breast.” That was a pathologist’s terminology for breast cancer. Now doctors know that one disease under a microscope is like seven or eight different molecular diseases if you look more deeply. There’s luminal A type, luminal B type, HER2 type, there’s triple negative type, and so on. And those different types behave differently with different chemotherapies. They also respond to specific therapies that are not available for the others. And that’s just breast cancer. The same kinds of things are true for other types of cancers as well as other diseases.
“In the 21st century, we’re looking more at these molecules and we’re understanding much more about how they contribute to disease, what they tell us about the prognosis of the patient, and what opportunities of therapy we can bring to bear,” LaBaer said.
The Human Genome Project, for the first time, outlined a complete human parts list. Looking at the human genome basically told us all the different genes that are there. That was the first step, and it was a big one. But that project looked at a few people’s genomes, and people vary widely.
The All of Us Research Program was launched by the U.S. government in 2018. It seeks to extend precision medicine to all diseases by building a national research cohort of 1 million or more U.S. participants. Anyone over the age of 18 living in the United States can join.
We all have a likelihood of getting different diseases. But when we do, our outcomes can differ from person to person with the same disease. Much of it is a product of our different genomes.
“How do we understand the variation?” LaBaer said. “What is the variation between us, and how does understanding that variation help predict risks of disease and/or responses to disease when they occur? By cataloging all that information, we will learn a lot about those sorts of factors. That’s what (All of Us) does for us."
There are limits to what genome info can do for disease risk. LaBaer’s favorite metaphor is the genome is a recipe, but people given the same recipe might make dishes that taste a little bit different.

"The genome is the starting point, but it’s not the answer to everything.” — Joshua LaBaer, professor and executive director of ASU’s Biodesign Institute.
The genome is the blueprint for how to make a person. People are a little different from the genome, because wear and tear happen to them. Things break. Sometimes people break even when they’ve always appeared to be fine, like a vegan athlete who develops diabetes in his late 40s.
“The genome doesn’t necessarily tell us what’s going to happen to a person,” LaBaer said. “It gives us the mathematical possibility of things that might happen to that person. … The genome can tell us likelihoods of our being able to metabolize certain drugs in certain ways. … That’s called pharmacogenomics, and that’s very important. The genome is the starting point, but it’s not the answer to everything.”
There are a lot of things about DNA information people need to know, LaBaer said. Although your entire human genome can be sequenced, fairly little is known about how to interpret that.
“If anyone tells you, ‘Oh, we’ll sequence your genome and that will fix everything,’ that’s probably not true,” he said. “It’s almost certainly not true. Certainly some of those elements are helpful. There are known genetic disorders you can detect.”
Whether you’re going to get heart disease or a specific type of cancer, mostly what’s now known can’t predict that. And, contrary to what you see on TV, genome sequencing can’t tell you whether your heritage is Albanian or Latvian. What do consumers need to watch out for?
“You need to be careful about what kind of promises are made about what you’re going to learn from this,” LaBaer said. “A lot of these companies initially promised all this medical value for people, and the FDA forced them to back away from that claim. Now most of them are marketing themselves as talking about your heritage. Even there, I think a lot of what’s promised is a little bit oversold at this point. When people say you’re 30 percent this and 15 percent that, I don’t know what that means. I don’t know how well that’s understood at this point. … DNA is only useful if the clinical information attached to it is also accurate. Oftentimes it isn’t.”
LaBaer cautions it’s worth looking at the fine print for privacy issues. Some of the companies sequencing genomes are selling that information to other companies for research purposes. Theoretically it’s not identified as yours. They’ll say it’s from a Caucasian female in her 30s, or something along those lines. A lot of their business models aren’t based on the fees you paid, but fees from selling the sequence to someone else. And, as is discussed in other sections of this series , there are no legal barriers from law enforcement going in to any of these companies and seeing what they have.
Finding solutions with gene therapies
When the gene editing tool CRISPR burst upon the scene in 2012, scientists immediately saw its potential to cure genetic diseases. Samira Kiani has built her career around her passion for applying CRISPR technology to synthetic biology. An assistant professor in the School of Biological and Health Systems Engineering, she has established her research program to combine CRISPR technology with synthetic biology to develop safer and controllable gene therapies.

Samira Kiani
Is that potential realistic? How viable are solutions?
There are three major areas CRISPR can potentially make an impact, according to Kiani. The first is gene therapy: Patients with formal genetic diseases like metabolic diseases or immune disorders have some sort of faulty genes.
“We can use CRISPR to disrupt those faulty genes or correct those faulty genes,” Kiani said. “This time CRISPR would allow us to pinpoint the type of genes that already exist in human DNA and just modify those, correct those or disrupt the faulty genes.”
Another potential arena for CRISPR would lie in correcting susceptibility genes that put people at risk of diseases like diabetes, cancer and atherosclerosis. A delivery device would put CRISPR in the patient’s body. The tool would go to a certain organ and change the genes.
“CRISPR would allow us at some point — let’s say five or 10 years from now — to develop a form of gene therapy using CRISPR and go and modulate those genes so that they are not really conferring susceptibility anymore to those diseases,” Kiani said.
The third application for human health Kiani cites is correcting a faulty gene at the embryonic level. For example, if a couple had genes that would immediately lead to a fetal disease, they could do in vitro fertilization and the genes could be corrected at the level of the embryo. Then the corrected embryo could be implanted.
CRISPR also is being used to diagnose certain genetic diseases or viruses that can infect cells such as HPV, HIV or Ebola.
Clinical applications are feasible within five to 10 years, according to Kiani. The technology is moving rapidly — but there’s a catch.
Science fiction writer William Gibson famously said, “The future is here. It’s just not widely distributed yet.” Travel from a big city to a rural town, or from an industrialized nation to a developing one, and unequal distribution of advanced anything is obvious.
“With technologies like this, you will face all the issues with access and equality of access,” Kiani said. “How do we make it affordable for every doctor’s office to have it? If we are speaking with regard to accessibility to patients at every doctor’s office, I would say a longer term — maybe 15 or 20 years. As any new technology is developed — internet technology or iPhone — every time these new technologies develop, rich (people) have better access to it. So I would say once this technology is rapidly developed, it’s either accessible to people with more money or governments and insurance companies need to come on board so they actually provide this accessibility to patients.”
Spinal muscular atrophy is a debilitating, muscle-wasting disease caused by death of nerve cells in the spine. The FDA approved the sale of a new drug for the treatment of this disease. The drug tricks the spinal neurons into using another gene to produce protein, allowing the patient to survive. Here’s the catch: The drug costs $750,000 in the first year followed by $375,000 a year after that — for life.
Gene therapies have the potential to alleviate that problem of cost. They require the creation of a drug specific for each patient. It has to be designed, customized, administered and monitored by several expert personnel. Currently, none of that comes cheap.
But there is a light at the end of that tunnel, Kiani said.
“The claim with CRISPR is because it’s easier to repurpose, the costs might be lower,” she said.
We can — but should we?
Ethical questions concerning biotechnology were already a part of the science and health policy conversation by the time the field of human genetics took off, thanks in part to biological weapons research that lasted until the Biological Weapons Convention in 1972 and the advent of agricultural biotechnology (which remains controversial to this day).
In relation to DNA science, School of Life Sciences Associate Professor Ben Hurlbut said ethical concerns arose out of the combination of the hopes that were attached to what knowledge the human genome could give us — such as the capacity to treat disease — and the uses it might be put to that could be contrary to the public good.
Hurlbut and colleagues are working on creating a new kind of structure for governance of the field — a global observatory for gene editing, which he wrote about in a March 2018 article for Nature.
“In the earliest days of the development of genetics and the technology associated with it, there was a tendency in the scientific community to ask those large ethical questions,” he said. “But over the years, there’s been a kind of resistance to that and a silencing of discussions that look far ahead.”
Cook-Deegan can attest to the former. A few years into working on the Human Genome Project, he authored “ The Gene Wars: Science, Politics, and the Human Genome ,” a personal account of the genesis and early stages of the project that also addressed anxieties regarding far-reaching medical and social implications. Later, he would go on to found Duke University’s Center for Genome Ethics, Law and Policy.
What is interesting about the field of human genetics, he noted, is that it started to take off at the same time that historians around the world were beginning to re-examine the history of eugenics and so-called "racial hygiene" that led to sterilization and interracial marriage bans. So as the field advanced, so too did unease about such ills resurfacing.
At the same time, most understood the potential health benefits of genomics.
“So from the beginning, there were ethical discussions and a parallel effort to do something about policy, to think about the legal issues that were going to need to be addressed,” Cook-Deegan said.
Some of the earliest ethical concerns with biotechnology were related to biosafety, military and industrial control of life and genetic engineering. Lately, as Hurlbut mentioned, things have become even more complicated.

“Our ability to do stuff far exceeds our ability to do it ethically.” — Andrew Maynard , professor in the School for the Future of Innovation in Society
In 2013, in response to a molecular diagnostic company that attempted to do so, the Supreme Court ruled that isolated human genes could not be patented. While proponents of the argument claimed patents would encourage investment in biotechnology and promote innovation in genetic research, opponents claimed patenting isolated genes would hamper further disease research and limit options for patients seeking genetic testing.
And there’s also reason to question whether we rely too much on what DNA tells us about disease risk factors to determine treatments and predict health outcomes.
“I'm not an MD,” Wilson said, “but for example, aspirin is advised to give to everyone to help prevent stroke. Turns out, it doesn't really work in women. And this has been known for decades. But we just give it to them anyway.
“So we have personalized medicine based on populations that are not representative of the people we're working on. If we really want to have personalized medicine, we need to actually have our data sets be representative of everyone. And they're not right now, unfortunately.”
Andrew Maynard , professor in the School for the Future of Innovation in Society, studies emerging tech and responsible innovation. In his new book, “ Films from the Future ,” he grapples with a number of issues around the ethics of how we work with DNA and what it means to innovate responsibly.
In the years to come, he believes there is a growing urgency for not just scientists but everyone DNA technology has the potential to affect to learn how to be socially responsible with it.
“Our ability to do stuff far exceeds our ability to do it ethically,” he said. “So there’s a huge obligation for us to think critically about what we’re doing and have an open conversation about it.”
Gene modification on our tables
As for that controversial agricultural biotechnology, genetically modified organisms have been around since the early 1970s. Definitions vary, but consensus hovers around an organism that has been altered in a way that would not occur in nature.
A bacteria was the first organism to have its DNA altered, followed by a mouse and a plant. The first organism engineered for commercial ends was the Flavr Savr tomato , which hit supermarket shelves in 1994. The FDA declared it as safe as a natural tomato. The goal of all tomato growers is to be able to handle them as soon as possible and for them to have a longer shelf life. The maker’s intent was to slow down ripening. Flavr Savrs did have a longer shelf life, but they still had to be picked and handled like any vine-ripened tomato. The company struggled with profits, mainly because they didn’t know enough about the farming end of the business, and were eventually acquired by Monsanto.
Flash-forward another decade and GloFish hit the market. They’re still around, for people who think tropical fish are too drab. In 2015, AquAdvantage Atlantic salmon hit Canadian markets. Modified to grow to market size in 16 to 18 months instead of three years, it was initially blocked from being sold in the U.S. In early March, however, the FDA lifted the import ban on genetically engineered salmon and salmon eggs.
Oya Yazgan is a molecular biologist in the College of Integrative Sciences and Arts, where she teaches a course in food and human health. How foods are produced and the consequences of consuming various types of foods is her passion.
There's one big question hovering over GMO foods: Are they safe? The short answer — no one really knows. Research has been done and used as a reference for saying that GMOs are safe, but it’s neither serious nor reliable science, Yazgan said.
"We need to take a very careful look at these before we play with people’s health." — Oya Yazgan, molecular biologist in the College of Integrative Sciences and Arts
“The studies they refer to are poorly designed and statistical analyses are not strong, and they are making conclusions that are not scientifically valid,” she said. “We have some preliminary evidence that needs stronger scientific research that indicates there are damages that are being caused by these GMOs. They are seeing intestinal damage in mice and pigs. The general bigger problem I see is that these studies are not designed well. They are very short-term, when you think about any possible effects. They are truncating these studies. If you don’t see the effects, then they are concluding that these are safe, which is, in my opinion and many other people’s opinions, irresponsible.”

Studies concluding GMOs are safe often have been conducted by industry-sponsored researchers. Independent researchers have an opposite view.
“A lot of publications and news reports and everything that I look at basically has ties to industry,” Yazgan said. “This is a huge industry — everyone is aware of that — and the feeling is that this is being pushed before we have definitive answers about their safety. That is my concern and my frustration about this as well.”
GMO foods are clearly labeled as such in the European Union. In the U.S., food is either organic or it’s not.
“There is that push because industry has a stronger hold on scientific research and the publications and what’s being made available to the public,” Yazgan said. “In Europe there are more regulations controlling the release of these GMOs and any other substance as well. There is more public support in Europe. There is more business support in the U.S. That’s the biggest difference.”
What’s the best option for concerned consumers? Right now that would be organic, because GMOs aren’t labeled. Big agriculture is trying to wiggle its way out of regulations, Yazgan said.
“The latest technique that is used to make modifications in the genes, they are little different from the previous ones and they do not leave a mark on the DNA of the organisms they are changing,” she said. “The FDA does not consider that genetically engineered, even though they are. They are trying to avoid the regulations.”
Intestinal problems, like irritable bowel syndrome, are on the rise, but not definitively linked to GMOs.
“We need to take a very careful look at these before we play with people’s health,” Yazgan said.
Written by Emma Greguska and Scott Seckel/ASU Now
More stories in this series
- DNA enters legal maze with potential
- How criminal justice is evolving with DNA
- Ask a Biologist's DNA primer
- Proofreading the book of life: Gene editing made safer
- Anthropology meets genetics to tell our collective story
More Science and technology

'More to munch on': The 'popcorn planet' unveils new atmospheric details
The "popcorn planet" is back in the spotlight.Using NASA's James Webb Space Telescope (JWST), a team of international astronomers has discovered new atmospheric details on WASP-107 b, an exoplanet…

ASU researchers contribute to study investigating how microbes behave in reduced gravity
Microbes populate every corner of the Earth and accompany humans wherever they travel, including space. Microbes also play a critical role in maintaining the balance between health and disease.…

ASU making its mark across the universe
By Wendee NicoleThe 3D model of the Psyche asteroid that sits on ASU Regents Professor Lindy Elkins-Tanton’s desk, a gray elliptical “potato” with craters, is the best rendition possible without ever…
This page has been archived and is no longer updated
Discovery of DNA Structure and Function: Watson and Crick
Many people believe that American biologist James Watson and English physicist Francis Crick discovered DNA in the 1950s. In reality, this is not the case. Rather, DNA was first identified in the late 1860s by Swiss chemist Friedrich Miescher. Then, in the decades following Miescher's discovery, other scientists--notably, Phoebus Levene and Erwin Chargaff--carried out a series of research efforts that revealed additional details about the DNA molecule, including its primary chemical components and the ways in which they joined with one another. Without the scientific foundation provided by these pioneers, Watson and Crick may never have reached their groundbreaking conclusion of 1953: that the DNA molecule exists in the form of a three-dimensional double helix .
The First Piece of the Puzzle: Miescher Discovers DNA
Although few people realize it, 1869 was a landmark year in genetic research, because it was the year in which Swiss physiological chemist Friedrich Miescher first identified what he called "nuclein" inside the nuclei of human white blood cells. (The term "nuclein" was later changed to " nucleic acid " and eventually to " deoxyribonucleic acid ," or "DNA.") Miescher's plan was to isolate and characterize not the nuclein (which nobody at that time realized existed) but instead the protein components of leukocytes (white blood cells). Miescher thus made arrangements for a local surgical clinic to send him used, pus-coated patient bandages; once he received the bandages, he planned to wash them, filter out the leukocytes, and extract and identify the various proteins within the white blood cells. But when he came across a substance from the cell nuclei that had chemical properties unlike any protein, including a much higher phosphorous content and resistance to proteolysis (protein digestion), Miescher realized that he had discovered a new substance (Dahm, 2008). Sensing the importance of his findings, Miescher wrote, "It seems probable to me that a whole family of such slightly varying phosphorous-containing substances will appear, as a group of nucleins, equivalent to proteins" (Wolf, 2003).
More than 50 years passed before the significance of Miescher's discovery of nucleic acids was widely appreciated by the scientific community. For instance, in a 1971 essay on the history of nucleic acid research, Erwin Chargaff noted that in a 1961 historical account of nineteenth-century science, Charles Darwin was mentioned 31 times, Thomas Huxley 14 times, but Miescher not even once. This omission is all the more remarkable given that, as Chargaff also noted, Miescher's discovery of nucleic acids was unique among the discoveries of the four major cellular components (i.e., proteins, lipids, polysaccharides, and nucleic acids) in that it could be "dated precisely... [to] one man, one place, one date."
Laying the Groundwork: Levene Investigates the Structure of DNA
Meanwhile, even as Miescher's name fell into obscurity by the twentieth century, other scientists continued to investigate the chemical nature of the molecule formerly known as nuclein. One of these other scientists was Russian biochemist Phoebus Levene. A physician turned chemist, Levene was a prolific researcher, publishing more than 700 papers on the chemistry of biological molecules over the course of his career. Levene is credited with many firsts. For instance, he was the first to discover the order of the three major components of a single nucleotide (phosphate-sugar-base); the first to discover the carbohydrate component of RNA (ribose); the first to discover the carbohydrate component of DNA (deoxyribose); and the first to correctly identify the way RNA and DNA molecules are put together.
During the early years of Levene's career, neither Levene nor any other scientist of the time knew how the individual nucleotide components of DNA were arranged in space; discovery of the sugar-phosphate backbone of the DNA molecule was still years away. The large number of molecular groups made available for binding by each nucleotide component meant that there were numerous alternate ways that the components could combine. Several scientists put forth suggestions for how this might occur, but it was Levene's "polynucleotide" model that proved to be the correct one. Based upon years of work using hydrolysis to break down and analyze yeast nucleic acids, Levene proposed that nucleic acids were composed of a series of nucleotides, and that each nucleotide was in turn composed of just one of four nitrogen-containing bases, a sugar molecule, and a phosphate group. Levene made his initial proposal in 1919, discrediting other suggestions that had been put forth about the structure of nucleic acids. In Levene's own words, "New facts and new evidence may cause its alteration, but there is no doubt as to the polynucleotide structure of the yeast nucleic acid" (1919).
Indeed, many new facts and much new evidence soon emerged and caused alterations to Levene's proposal. One key discovery during this period involved the way in which nucleotides are ordered. Levene proposed what he called a tetranucleotide structure, in which the nucleotides were always linked in the same order (i.e., G-C-T-A-G-C-T-A and so on). However, scientists eventually realized that Levene's proposed tetranucleotide structure was overly simplistic and that the order of nucleotides along a stretch of DNA (or RNA) is, in fact, highly variable . Despite this realization, Levene's proposed polynucleotide structure was accurate in many regards. For example, we now know that DNA is in fact composed of a series of nucleotides and that each nucleotide has three components: a phosphate group ; either a ribose (in the case of RNA) or a deoxyribose (in the case of DNA) sugar; and a single nitrogen-containing base. We also know that there are two basic categories of nitrogenous bases: the purines ( adenine [A] and guanine [G]), each with two fused rings, and the pyrimidines ( cytosine [C], thymine [T], and uracil [U]), each with a single ring. Furthermore, it is now widely accepted that RNA contains only A, G, C, and U (no T), whereas DNA contains only A, G, C, and T (no U) (Figure 1).
Strengthening the Foundation: Chargaff Formulates His "Rules"
Erwin Chargaff was one of a handful of scientists who expanded on Levene's work by uncovering additional details of the structure of DNA, thus further paving the way for Watson and Crick. Chargaff, an Austrian biochemist, had read the famous 1944 paper by Oswald Avery and his colleague s at Rockefeller University, which demonstrated that hereditary units, or genes , are composed of DNA. This paper had a profound impact on Chargaff, inspiring him to launch a research program that revolved around the chemistry of nucleic acids. Of Avery's work, Chargaff (1971) wrote the following:
"This discovery, almost abruptly, appeared to foreshadow a chemistry of heredity and, moreover, made probable the nucleic acid character of the gene ... Avery gave us the first text of a new language, or rather he showed us where to look for it. I resolved to search for this text."
As his first step in this search, Chargaff set out to see whether there were any differences in DNA among different species . After developing a new paper chromatography method for separating and identifying small amounts of organic material, Chargaff reached two major conclusions (Chargaff, 1950). First, he noted that the nucleotide composition of DNA varies among species. In other words, the same nucleotides do not repeat in the same order, as proposed by Levene. Second, Chargaff concluded that almost all DNA--no matter what organism or tissue type it comes from--maintains certain properties, even as its composition varies. In particular, the amount of adenine (A) is usually similar to the amount of thymine (T), and the amount of guanine (G) usually approximates the amount of cytosine (C). In other words, the total amount of purines (A + G) and the total amount of pyrimidines (C + T) are usually nearly equal. (This second major conclusion is now known as "Chargaff's rule.") Chargaff's research was vital to the later work of Watson and Crick, but Chargaff himself could not imagine the explanation of these relationships--specifically, that A bound to T and C bound to G within the molecular structure of DNA (Figure 2).
Putting the Evidence Together: Watson and Crick Propose the Double Helix
Chargaff's realization that A = T and C = G, combined with some crucially important X-ray crystallography work by English researchers Rosalind Franklin and Maurice Wilkins, contributed to Watson and Crick's derivation of the three-dimensional, double-helical model for the structure of DNA. Watson and Crick's discovery was also made possible by recent advances in model building, or the assembly of possible three-dimensional structures based upon known molecular distances and bond angles, a technique advanced by American biochemist Linus Pauling. In fact, Watson and Crick were worried that they would be "scooped" by Pauling, who proposed a different model for the three-dimensional structure of DNA just months before they did. In the end, however, Pauling's prediction was incorrect.
Using cardboard cutouts representing the individual chemical components of the four bases and other nucleotide subunits, Watson and Crick shifted molecules around on their desktops, as though putting together a puzzle. They were misled for a while by an erroneous understanding of how the different elements in thymine and guanine (specifically, the carbon, nitrogen, hydrogen, and oxygen rings) were configured. Only upon the suggestion of American scientist Jerry Donohue did Watson decide to make new cardboard cutouts of the two bases, to see if perhaps a different atomic configuration would make a difference. It did. Not only did the complementary bases now fit together perfectly (i.e., A with T and C with G), with each pair held together by hydrogen bonds, but the structure also reflected Chargaff's rule (Figure 3).
Although scientists have made some minor changes to the Watson and Crick model, or have elaborated upon it, since its inception in 1953, the model's four major features remain the same yet today. These features are as follows:
- DNA is a double-stranded helix, with the two strands connected by hydrogen bonds. A bases are always paired with Ts, and Cs are always paired with Gs, which is consistent with and accounts for Chargaff's rule.
- Most DNA double helices are right-handed; that is, if you were to hold your right hand out, with your thumb pointed up and your fingers curled around your thumb, your thumb would represent the axis of the helix and your fingers would represent the sugar-phosphate backbone. Only one type of DNA, called Z-DNA , is left-handed.
- The DNA double helix is anti-parallel, which means that the 5' end of one strand is paired with the 3' end of its complementary strand (and vice versa). As shown in Figure 4, nucleotides are linked to each other by their phosphate groups, which bind the 3' end of one sugar to the 5' end of the next sugar.
- Not only are the DNA base pairs connected via hydrogen bonding, but the outer edges of the nitrogen-containing bases are exposed and available for potential hydrogen bonding as well. These hydrogen bonds provide easy access to the DNA for other molecules, including the proteins that play vital roles in the replication and expression of DNA (Figure 4).
One of the ways that scientists have elaborated on Watson and Crick's model is through the identification of three different conformations of the DNA double helix. In other words, the precise geometries and dimensions of the double helix can vary. The most common conformation in most living cells (which is the one depicted in most diagrams of the double helix, and the one proposed by Watson and Crick) is known as B-DNA . There are also two other conformations: A-DNA , a shorter and wider form that has been found in dehydrated samples of DNA and rarely under normal physiological circumstances; and Z-DNA, a left-handed conformation. Z-DNA is a transient form of DNA, only occasionally existing in response to certain types of biological activity (Figure 5). Z-DNA was first discovered in 1979, but its existence was largely ignored until recently. Scientists have since discovered that certain proteins bind very strongly to Z-DNA, suggesting that Z-DNA plays an important biological role in protection against viral disease (Rich & Zhang, 2003).
Watson and Crick were not the discoverers of DNA, but rather the first scientists to formulate an accurate description of this molecule's complex, double-helical structure. Moreover, Watson and Crick's work was directly dependent on the research of numerous scientists before them, including Friedrich Miescher, Phoebus Levene, and Erwin Chargaff. Thanks to researchers such as these, we now know a great deal about genetic structure, and we continue to make great strides in understanding the human genome and the importance of DNA to life and health.
References and Recommended Reading
Chargaff, E. Chemical specificity of nucleic acids and mechanism of their enzymatic degradation. Experientia 6 , 201–209 (1950)
---. Preface to a grammar of biology. Science 171 , 637–642 (1971)
Dahm, R. Discovering DNA: Friedrich Miescher and the early years of nucleic acid research. Human Genetics 122 , 565–581 (2008)
Levene, P. A. The structure of yeast nucleic acid. IV. Ammonia hydrolysis . Journal of Biological Chemistry 40 , 415–424 (1919)
Rich, A., &. Zhang, S. Z-DNA: The long road to biological function. Nature Reviews Genetics 4 , 566–572 (2003) ( link to article )
Watson, J. D., & Crick, F. H. C. A structure for deoxyribose nucleic acid. Nature 171 , 737–738 (1953) ( link to article )
Wolf, G. Friedrich Miescher: The man who discovered DNA. Chemical Heritage 21 , 10-11, 37–41 (2003)
- Add Content to Group
Article History
Flag inappropriate.


Email your Friend

- | Lead Editor: Bob Moss

Within this Subject (34)
- Applications in Biotechnology (4)
- Discovery of Genetic Material (4)
- DNA Replication (6)
- Gene Copies (5)
- Jumping Genes (4)
- RNA (7)
- Transcription & Translation (4)
Other Topic Rooms
- Gene Inheritance and Transmission
- Gene Expression and Regulation
- Nucleic Acid Structure and Function
- Chromosomes and Cytogenetics
- Evolutionary Genetics
- Population and Quantitative Genetics
- Genes and Disease
- Genetics and Society
- Cell Origins and Metabolism
- Proteins and Gene Expression
- Subcellular Compartments
- Cell Communication
- Cell Cycle and Cell Division
© 2014 Nature Education
- Press Room |
- Terms of Use |
- Privacy Notice |

Visual Browse
- Search Menu
- Sign in through your institution
- Advance Articles
- Perspectives
- Knowledgebase and Database Resources
- Nobel Laureates Collection
- China Virtual Outreach Webinar
- Neurogenetics
- Fungal Genetics and Genomics
- Multiparental Populations
- Genomic Prediction
- Plant Genetics and Genomics
- Genetic Models of Rare Diseases
- Genomic Data Analyses In Biobanks
- Genetics of Bacteria
- Why Publish
- Author Guidelines
- Submission Site
- Open Access Options
- Full Data Policy
- Self-Archiving Policy
- About Genetics
- About Genetics Society of America
- Editorial Board
- Early Career Reviewers
- Guidelines for Reviewers
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Some dna repair mechanisms, replication as the central problem in biology, the “stability of dna”, the contribution of radiation biology, excision repair, base excision and mismatch repair, acknowledgments, literature cited.
- < Previous
Why Is DNA Double Stranded? The Discovery of DNA Excision Repair Mechanisms
- Article contents
- Figures & tables
- Supplementary Data
Bernard S Strauss, Why Is DNA Double Stranded? The Discovery of DNA Excision Repair Mechanisms, Genetics , Volume 209, Issue 2, 1 June 2018, Pages 357–366, https://doi.org/10.1534/genetics.118.300958
- Permissions Icon Permissions
The double stranded structure of DNA suggested a mechanism for replication. Overlooked was that it also served to maintain genome stability by providing a template for the repair of damage and mistakes in replication...
The persistence of hereditary traits over many generations testifies to the stability of the genetic material. Although the Watson–Crick structure for DNA provided a simple and elegant mechanism for replication, some elementary calculations implied that mistakes due to tautomeric shifts would introduce too many errors to permit this stability. It seemed evident that some additional mechanism(s) to correct such errors must be required. This essay traces the early development of our understanding of such mechanisms. Their key feature is the cutting out of a section of the strand of DNA in which the errors or damage resided, and its replacement by a localized synthesis using the undamaged strand as a template. To the surprise of some of the founders of molecular biology, this understanding derives in large part from studies in radiation biology, a field then considered by many to be irrelevant to studies of gene structure and function. Furthermore, genetic studies suggesting mechanisms of mismatch correction were ignored for almost a decade by biochemists unacquainted or uneasy with the power of such analysis. The collective body of results shows that the double-stranded structure of DNA is critical not only for replication but also as a scaffold for the correction of errors and the removal of damage to DNA. As additional discoveries were made, it became clear that the mechanisms for the repair of damage were involved not only in maintaining the stability of the genetic material but also in a variety of biological phenomena for increasing diversity, from genetic recombination to the immune response.
THE Austrian theoretical physicist, Erwin Schrödinger, one of the inventors of wave mechanics, was fascinated by the Hapsburg lip, a distinctive facial feature of the Hapsburg imperial family. This was not only because he was Austrian but, as a physicist trying to understand biology, he was fascinated by the stability of this trait over the centuries, something that seemed to defy the laws of thermodynamics ( Schrödinger 1945 ). Geneticists and biochemists in the 1940s were comparably impressed by the apparent removal of DNA from the hurly-burly of cellular metabolism, a property that one might associate with such hereditary stability ( Mazia 1952 ).
A major step forward in understanding the properties of the genetic material was the formulation of the double-stranded structure of DNA by James Watson and Francis Crick in 1953, which suggested a mechanism for its replication and accordingly its perpetuation. In one of the more famous understatements in the scientific literature they wrote: “It has not escaped our attention that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material” ( Watson and Crick 1953a , b ). What apparently did escape their attention, and that of the early molecular biologists, was that this double-stranded structure also served as a safety device, permitting the repair of damage to one or the other of the strands. Even more surprising, in hindsight at least, was that this recognition first came from what was then the unfashionable field of radiation biology.
Today the subject of DNA repair is a fully accepted part of the body of contemporary molecular knowledge. Current textbooks of molecular biology, genetics, and biochemistry list DNA repair mechanisms comfortably among the multitude of metabolic pathways. Table 1 summarizes the ones discussed in this article. Manipulation of these pathways is central to the application of CRISPR, perhaps the most productive of recent biological technologies and the latest major addition to the field of DNA repair. The Nobel Prize in chemistry for 2015 was awarded to Tomas Lindahl, Paul Modrich, and Aziz Sancar for their detailed mechanistic studies on repair, which is confirmation of the current respectability of studies on DNA repair.
| Type . | Substrate . | Double-stranded DNA required . | Mechanism . | Key reference(s) and/or reviews . |
|---|---|---|---|---|
| Nucleotide excision repair | UV-induced pyrimidine dimers, bulky adducts | Yes | Distortions in DNA helix are recognized. Nucleases cut the nucleotide chain above and below the damage. DNA helicase removes a 12-nt segment. The gap is filled in by DNA polymerase and ligase. | ; ; ; |
| Transcription-coupled NER | As above | Yes | A variant of NER. Acts at site of stalled RNA polymerase | (1987) |
| Base excision repair | Unusual or mispairing damaged bases (oxidized, methylated, deaminated, uracil, single-strand breaks) | Yes | DNA glycosylases remove bases forming abasic sites which are cleaved by an endonuclease. The single-strand break is processed and a patch of from 1 to 2–10 nt is inserted. | |
| Mismatch repair | Mismatched bases produced by replication or recombination | Yes | Mismatches are detected in the newly synthesized strand by methylation of the parent strand in enteric bacteria, single-strand nicks in other species. The mismatched strand is then cleaved and a segment of variable length including the mismatch is removed. The single-strand gap is repaired by the replicative polymerase and sealed by ligase. | ; |
| Double-strand break repair, homologous recombination | DNA with both strands broken by external agents ( , radiation) or during genetic recombination | Yes (a homologous chromosome or chromatid) | The double-strand break is enlarged by nucleases to leave overhanging single strands. An undamaged strand from the double-stranded homolog pairs with the resected region and serves as a template for DNA synthesis. The crossed over strands form a Holliday junction which can be resolved in different ways. | (1983) |
| Nonhomologous end joining | DNA with both strands broken by external agents or during the immune response | ? (short microhomologies) | The broken ends are brought together with the deletion or addition of a few bases and then patched together by specific proteins. | |
| Photoreactivation | DNA with pyrimidine dimers produced by UV radiation | No? | The bonds connecting adjacent pyrimidines are enzymatically broken using energy from visible light. The repaired strand is not broken. | ; |
| Methyl removal from O methylguanine | O methylguanine in DNA | No | The methyl from O methylguanine is transferred to O methylguanine DNA transferase. The protein is inactivated as a result. The repaired strand is not broken. | (1979) |
| Type . | Substrate . | Double-stranded DNA required . | Mechanism . | Key reference(s) and/or reviews . |
|---|---|---|---|---|
| Nucleotide excision repair | UV-induced pyrimidine dimers, bulky adducts | Yes | Distortions in DNA helix are recognized. Nucleases cut the nucleotide chain above and below the damage. DNA helicase removes a 12-nt segment. The gap is filled in by DNA polymerase and ligase. | ; ; ; |
| Transcription-coupled NER | As above | Yes | A variant of NER. Acts at site of stalled RNA polymerase | (1987) |
| Base excision repair | Unusual or mispairing damaged bases (oxidized, methylated, deaminated, uracil, single-strand breaks) | Yes | DNA glycosylases remove bases forming abasic sites which are cleaved by an endonuclease. The single-strand break is processed and a patch of from 1 to 2–10 nt is inserted. | |
| Mismatch repair | Mismatched bases produced by replication or recombination | Yes | Mismatches are detected in the newly synthesized strand by methylation of the parent strand in enteric bacteria, single-strand nicks in other species. The mismatched strand is then cleaved and a segment of variable length including the mismatch is removed. The single-strand gap is repaired by the replicative polymerase and sealed by ligase. | ; |
| Double-strand break repair, homologous recombination | DNA with both strands broken by external agents ( , radiation) or during genetic recombination | Yes (a homologous chromosome or chromatid) | The double-strand break is enlarged by nucleases to leave overhanging single strands. An undamaged strand from the double-stranded homolog pairs with the resected region and serves as a template for DNA synthesis. The crossed over strands form a Holliday junction which can be resolved in different ways. | (1983) |
| Nonhomologous end joining | DNA with both strands broken by external agents or during the immune response | ? (short microhomologies) | The broken ends are brought together with the deletion or addition of a few bases and then patched together by specific proteins. | |
| Photoreactivation | DNA with pyrimidine dimers produced by UV radiation | No? | The bonds connecting adjacent pyrimidines are enzymatically broken using energy from visible light. The repaired strand is not broken. | ; |
| Methyl removal from O methylguanine | O methylguanine in DNA | No | The methyl from O methylguanine is transferred to O methylguanine DNA transferase. The protein is inactivated as a result. The repaired strand is not broken. | (1979) |
A listing of (human) proteins involved in DNA repair processes can be found in Wood et al. (2005) . NER, nucleotide excision repair.
Yet it is clear that the early workers in this field were justified in feeling that their work was not given the recognition it deserved as a key factor in the DNA-centered view of life that became the science of molecular biology. John Cairns, a key figure in that development, writing as late as in 2008, was able to trace the foundations of molecular biology and list its exciting discoveries without mentioning the fact that DNA could be repaired ( Cairns 2008 ). A review on the history of “target theory” (a pioneering, somewhat earlier, attempt to understand the biological effects of radiation) ( Box 1 ) reports: “Around 30 years ago, a very prominent molecular biologist confidently proclaimed that nothing of fundamental importance has ever been learned by irradiating cells!” ( Bedford and Dewey 2002 ; J. S. Bedford, personal communication). What was the basis for this attitude and what produced the change?
The introduction of ionizing radiation as a tool in the 1920s and 1930s led to major advances in our understanding of the gene. The discovery by Muller (1927) and almost simultaneously by Stadler (1928a , b ) that ionizing radiation could produce mutations in what had hitherto been an impenetrable gene opened up the possibility of actually investigating the properties of this biological entity by physical means. Further investigations by Timofeeff-Ressovsky et al. (1935) led to the hypothesis that the “gene” was a molecule and to a calculation of its possible size that was/is reasonable. This “three-man article” found its way to Schrödinger (1945) who made Delbrück’s model a key feature of his book What is Life? , a work which enticed many of its founders into what became molecular biology.
The advantage and disadvantage of radiation is that it lent itself to quantitative studies and to a mathematical analysis of the results obtained. The result was target theory: the idea that the gene, or virus, was a target at which quantum bullets could be shot. There was then a relationship between the size of the target and the number (dose) of bullets that needed to be shot at random to hit the target. The hypothesis was reasonable as a first approximation and was developed to a high degree of sophistication ( Lea 1946 ). The hypothesis had many failings but, to my mind, a major one was the concentration of research on the absolute linear dependence of the mutation rate on dose. There were political and social reasons for this concentration in a world attempting to come to terms with the development of atomic energy. One scientific result was a concentration of radiation research on the interpretation of killing curves with different types of radiation being applied at differing dose rates and with different end points. At no point was this research able to identify the target molecule. Notwithstanding really sophisticated analysis, this research did not provide as much insight as subsequent biochemical analysis.
In the 1930s, Max Delbrück, a brilliant young German physicist, became interested in radiation biology as a tool for discerning the nature of the gene ( Strauss 2017 ). He was looking for some way to validate Niels Bohr’s speculation that understanding biology required the recognition of unique processes that could not be explained by the application of (known) physical and chemical principles ( Bohr 1933 ). Replication of the genetic material appeared to be the most likely place in which such new principles might be found. Delbrück’s work on bacterial viruses started with the expectation that these entities might be the simplest objects to study “pure” replication without the distractions of metabolism. Unfortunately, the viruses turned out to be not nearly as simple as Delbrück had imagined. They possessed multiple genes, a complex recombination mechanism, and even a sequential developmental pattern. Accordingly, the problem of replication remained.
At almost the same time as these investigations on bacteriophage were beginning, Oswald Avery and his co-workers were demonstrating that (at least some) genetic information was carried by DNA ( Avery et al. 1944 ). By 1952 it was generally recognized that it was DNA rather than protein that carried the genetic message ( Mazia 1952 ). The culmination of these efforts was the elucidation of the DNA structure by Watson and Crick (1953a , b ).
Crick, at least in hindsight, recognized that their proposed mechanism had a potentially fatal flaw ( Crick 1974 ). DNA could not be the carrier of stable genetic information since the calculated rate of errors in its replication based on the rate of tautomeric shifts in the nucleotide bases would make such hereditary stability impossible. Yet, in spite of that theoretical objection, DNA is the genetic material. Therefore, there must be mechanisms for correcting errors introduced in the normal replication process.
For most of the founders of molecular biology, the exact nature of such mechanisms were just secondary details that could be worked out later compared with the really important questions of how DNA replicated and functioned. Their rationale was outlined by Crick much later ( Crick 1974 , 1988 ):
Surely then, DNA cannot be the genetic material since its replication would produce too many errors.... Fortunately, we never took this argument seriously…. DNA is, in fact, so precious and so fragile that we now know that the cell has evolved a whole variety of repair mechanisms to protect its DNA from assaults by radiation, chemicals and other hazards… ( Crick 1988 , p. 111).
This clearly reflects the wisdom of hindsight. Replication using complementary base pairing is possible without a permanent double-stranded DNA structure: consider the single-stranded viruses. However, the stability of the genome requires repair and at least four separate pathways—nucleotide excision repair and its variation, transcription-coupled nucleotide excision repair, base excision repair, and mismatch repair—have evolved to accomplish it ( Table 1 ).
By 1952, as noted above, it was pretty well established that DNA was the genetic material. A review by Mazia (1952) summarizes the reasons for this belief. Mazia emphasized the finding that DNA, but not protein, met the quantitative expectations for the hereditary substance. The amount of DNA was the same in all diploid cells and was halved in haploid cells. The DNA content of the nucleus (exactly) doubled during the mitotic cycle. An interesting additional argument was based on the apparent stability of DNA. It had recently been shown that body constituents were in a constant state of turnover ( Schoenheimer 1942 ), but there was an important exception. DNA seemed to be different and unusually stable. Its constituent atoms did not seem to be replaced to the same extent as other molecules, particularly RNA (then called PNA for pentose nucleic acid) ( e.g. , Furst et al. 1950 ). This was in accord with the (supposed) requirements of the genetic material, the guardian of the cell’s history, protected from the vicissitudes of metabolism, and able to remain stable for hundreds of years as Schrödinger had pointed out in his 1945 essay.
Mazia wrote his review just before publication of either the Hershey–Chase experiment ( Hershey and Chase 1952 ) or the Watson and Crick model ( Watson and Crick 1953b ). He was aware of the Avery experiments ( Avery et al. 1944 ) but, like most cell physiologists, was not sure what to make of experiments with bacteria. Mazia was reasonably sure that transformation was not directed mutation and thought it unlikely to be due to protein contamination, but he was not sure where the specificity of DNA came from. He quotes Chargaff’s work showing the different base composition of DNAs from various bacteria ( Chargaff 1950 ), but he also quotes work from Mirsky’s laboratory ( Daly et al. 1950 ) showing that the DNAs from a variety of vertebrates and Pneumococcus had essentially the same base composition. Nonetheless, Mazia came down on the side of DNA as the genetic material and identified what he saw as the two remaining problems: how did DNA reproduce and how did it function? The Watson–Crick structure pointed to the solution of the first question. The second was Crick’s major question (how genes functioned) and occupied him and many prominent molecular biologists for the decades of the 1950s and 1960s. The discovery of messenger RNA, elucidation of the role of repressors and promoters, and an increased understanding of how DNA coded information early in the 1960s made it possible to think that Crick’s second question had been solved in principle.
In parallel with these developments, but carried out by a separate set of investigators, were developments in the study of mutation. It was demonstrated by bona fide members of the “phage group” that a variety of nucleic acid base analogs could induce mutations in phage, and Ernst Freese and Seymour Benzer had developed a molecular explanation for their action ( Benzer and Freese 1958 ). The earlier pioneering work of Charlotte Auerbach ( Auerbach and Robson 1947 ; Auerbach et al. 1947 ) had spawned a series of experiments showing the production of mutations by chemical agents, particularly the alkylating agents. A group of investigators at The University of Texas had also demonstrated that UV irradiation of the medium made it mutagenic for bacteria, suggesting that at least some radiation effects might be indirect ( Stone et al. 1947 ). Furthermore, there was increasing evidence that protein synthesis was required to fix or “cement” mutations ( Witkin 1956 ). Therefore, by the mid-1950s, there was evidence indicating that biochemistry intervened between an insult to DNA and the production of a mutation.
The development of atomic weapons remains both a (perhaps “the”) major problem for our times and also represents the practical achievement of a half century of research in physics. This development could not help but affect biological research, and it did so in at least two ways. It certainly persuaded many physical scientists to look for a field they could pursue without qualms of conscience and it persuaded the United States government, anxious to promote the peaceful uses of atomic energy, to establish a number of National Laboratories and to spend relatively large sums, for those times, on biological research to ascertain the safety of such applications. Errol Friedberg (1997 ) and some other key investigators of DNA repair are convinced that “This situation did little to endear the community of radiobiologists to the ‘aristocrats’ of molecular biology, who not only labored under more restrictive financial conditions, but additionally considered much of the research done in these Laboratories as frankly pedestrian.” (The data don’t actually support the reality of the relative lavishness of support for radiation research but the feeling was certainly there.)
By 1960 there was an awareness that cells could recover from the effects of radiation even though the nature of the recovery process(es) was unknown. It was also recognized that any explanation of the origin and evolution of life would have to take into account damage to the genetic material from radiation in the prebiotic and early postbiotic eras. The astrophysicist Carl Sagan made some calculations for the Radiation Research Society and came to the conclusion that the major problem would be UV radiation ( Sagan 1961 ). The flux of UV light would have resulted in a mutation rate too high to permit stable transmission of hereditary information. Sagan’s 1961 calculations suggested that the original life forms would have to have been benthic, i.e. , living on the ocean bottom, but even then there would be a problem resulting from the production of peroxides. His suggestion as to how primitive organisms solved this problem reflected current knowledge and involved the development of catalases that would detoxify the peroxides. These speculations ignored the possibility that damaged DNA itself might be repaired. By the start of the 1960s it was recognized that radiation, particularly UV radiation, would have posed a significant problem for early (as well as later) organisms.
By this time there was sufficient information indicating genetic recovery processes to permit a Symposium to be held in Leiden in August 1962 with the title Repair from Genetic Radiation Damage ( Sobels 1963 ). By 1962 it was recognized that there was a repair process(es) acting on DNA and that this process acted in the dark and was distinct from a previously discovered light-dependent form of DNA repair: photoreactivation. The phenomenon of photoreactivation had been described in the late 1940s ( Kelner 1949 ) and had even been subsequently accomplished in vitro ( Wulff and Rupert 1962 ). It had been discovered that neighboring thymines in DNA were dimerized by UV radiation ( Beukers and Berends 1960 ; Wulff and Fraenkel 1961 ) and that these dimers disappeared during photoreactivation ( Wulff and Rupert 1962 ) (for review of this phenomenon see Friedberg 1997 ). In some ways, the discovery may have diverted attention from the role of the double-stranded DNA structure in repair, since photoreactivation involves the direct reversal of a lesion without breaking the DNA chain. The mechanism of the dark repair was unknown. No one at the 1962 Symposium recognized (or wrote about) the importance of having the DNA be double stranded to permit repair of any kind.
The discovery of thymine dimerization by radiation was the key to an understanding of how DNA could be repaired. Within 2–3 years from the report of the UV-induced dimerization of thymine, a general scheme of excision repair had been established as the result of work by Richard Setlow, Paul Howard-Flanders, Philip Hanawalt, and their co-workers. The demonstration depended on the isolation of radiation-sensitive mutants in Escherichia coli , first by Ruth Hill (1958 ) (who tragically died at an early age in 1973) and on some astute biochemical intuition by Richard Setlow about the properties and analysis of the excised thymine dimer-containing fragments ( Friedberg 1997 ). The precise timing of the discoveries by the three laboratories is not clear since the key articles were published within weeks of one another ( Boyce and Howard-Flanders 1964 ; Pettijohn and Hanawalt 1964 ; Setlow and Carrier 1964 ). An earlier result of Hanawalt ( Pettijohn and Hanawalt 1963 ) included evidence for what is now known as repair synthesis but without that interpretation. I believe that all subsequent studies on DNA repair mechanisms can be traced to these three articles. The elucidation of the general scheme of excision repair ( Figure 1 ) provided a paradigm for subsequent work.

Common steps in excision repair mechanisms.
At least one of the founders of molecular biology did immediately appreciate the importance of the discovery. Philip Hanawalt (personal communication) recalls that Max Delbrück was so excited about the discovery of thymine dimers that he decided to offer a course on photobiology and during one lecture he “became so excited that he lapsed into German and didn’t notice for a few minutes, before laughing and switching back to English.” Delbrück was the contributing editor of the Boyce–Howard-Flanders excision repair article. He was well known for his frequent, devastating comments at seminars ( Strauss 2017 ), but had a different attitude about the discovery of excision repair. In retrospect, this may not have been so strange. Delbrück had a lifelong interest in photobiology stemming from Niels Bohr’s lecture on Light and Life ( Bohr 1933 ), a lecture that influenced all his future work. I have written about Delbrück before ( Strauss 2017 ) but it is now clear to me that I had not sufficiently appreciated his interest in photobiology and repair. He moderated the final session of the first major meeting on DNA repair ( Haynes et al. 1965 ), but I did not then understand why he bothered to attend.
By 1967 the accumulating evidence for a generalized DNA repair mechanism was great enough to merit a Scientific American article describing the system and arguing for a generalized DNA repair mechanism ( Hanawalt and Haynes 1967 ). A great boon to research in the field was the discovery of a disease linked to cancer and resulting from a deficiency in DNA repair. This was the demonstration by James Cleaver that the sun-sensitive, cancer-prone genetic disease xeroderma pigmentosum was associated with a deficiency of DNA repair ( Cleaver 1968 ). Besides the obvious fundamental scientific importance of the discovery, it also established the health relevance of DNA repair, thereby providing an important rationale for securing financial support from the National Institutes of Health.
The fidelity of normal DNA replication is maintained by several processes. The overall error rate in vivo for undamaged DNA is ∼1 in 10 9 (error rates vary in different portions of the genome). Physical base pairing and stacking alone result in an error rate of ∼1 in 10 2 . Polymerase structure, the fitting of incoming nucleotides and template into the enzyme, contribute a factor of specificity of an additional 10 3 ( McCulloch and Kunkel 2008 ). Replicative polymerases have associated with them a “proofreading” exonuclease, which examines the incoming nucleotide for fit and which contributes an additional factor of ∼10 2 to the specificity ( Fersht et al. 1982 ). The additional factor of ∼10 2 for normal replication is contributed by the mismatch (excision) repair system. In addition, there are three excision repair mechanisms to deal with DNA damage and one distinct mechanism (homologous recombination) for the error-free repair of double-strand breaks in DNA ( Table 1 ). The pathways of the four excision repair mechanisms (including mismatch repair) are remarkably similar involving a recognition step, removal of the incorrect base, which involves a break in the phosphodiester chain, and a resynthesis step using the normal DNA strand as a template ( Figure 1 ). There is a second process for the repair of double-strand breaks (nonhomologous end joining) that is somewhat different but with a variant that does involve the two strands. To my knowledge, there has been no report of homologies in the proteins involved, which suggests that all have been independently evolved. However, it has been proposed that one or more of the mismatch repair proteins have been coopted to play a regulatory role in some of the excision repair mechanisms ( Mellon and Champe 1996 ; Polosina and Cupples 2010 ). The common theme of all the excision mechanisms is that DNA must be double stranded to provide a template for the repair. Separate from these, there are proteins involved in the direct reversal of damage ( e.g. , photoreactivation, O 6 methylguanine DNA methyl transferase).
Once the basic principles of nucleotide excision repair had been established, it became somewhat easier to think of variations on the process. For example, I had been studying the effect of the monofunctional alkylating agent, methyl methanesulfonate (MMS), on Bacillus subtilis and had observed a recovery process which, as a result of some convoluted arithmetical calculations, I attributed to residual DNA synthesis ( Strauss 1963 ). Although then a member of the Department of Microbiology at The University of Chicago, my laboratory was in the Research Institutes that also housed the Committee on Biophysics. Bob Haynes, who was a collaborator of Phil Hanawalt, was a member of that unit and we met more or less regularly at the mail boxes (this was in the period when “snail mail”—the regular postal delivery of paper—was still an important means of communication). Haynes was one of the organizers of the first meeting devoted to DNA repair held in Chicago in 1965 ( Haynes et al. 1965 ). One day I learned from Bob that there was a process called DNA repair. As a result, we then tested the different stocks of B. subtilis in our freezer and discovered that we had been using a UV-sensitive strain: origin unknown. We used this strain to show that there were variations in excision repair ( Reiter and Strauss 1965 ) since, along with other evidence, this strain did not repair UV-induced or nitrogen mustard-induced damage but did still repair MMS-induced damage. We concluded: “The repair of damage induced by ultraviolet irradiation differs by at least one step from the repair of damage induced by methyl methanesulphonate.” (I sent a copy of our manuscript to Richard Setlow asking for comment and he tried to dissuade me from this conclusion since it contradicted the hypothesis that excision repair was a general error-correcting mechanism.) In retrospect, what we were seeing, but did not understand, is now recognized as base excision repair; a system using different enzymes and a smaller patch.
Two other discoveries were needed to make the study of DNA repair processes part of the catechism of molecular biology. First, it needed to be shown that DNA repair was not a phenomenon limited to radiation- or alkylating agent-induced damage, but that there was a significant natural rate of damage or mismatch that required repair over and above the exonucleolytic proofreading activities of the replicative DNA polymerases. Second, it needed to be demonstrated that DNA recombination was a process that involved using some of the same tactics used for repair. To resolve Crick’s conundrum, the result of all the repair processes should be found to be a reduction of the overall replication error rate to a level consistent with the stability of the genome.
That some such system(s) was required was made empirically clear by a set of studies by Tomas Lindahl in the early 1970s. One of these studies was a measurement of the spontaneous loss of purines by DNA ( Lindahl and Nyberg 1972 ). Lindahl showed that, without any intervention whatsoever, a typical human cell might be expected to lose ∼600 purines/hr ( Lindahl and Nyberg 1972 ). This calculation, coupled with the discovery of an enzyme to remove spontaneously produced uracil from DNA ( Lindahl 1974 ), was evidence that the genetic material needed continuing surveillance and repair to maintain its integrity.
In hindsight, one can argue that the basic principles of mismatch repair could, and should, have been deduced by the early 1960s from the new data about the mechanisms of meiosis coming from the studies on Neurospora and yeast. In these studies, all the products of an individual meiosis could be recovered; hence, in these organisms it was possible to follow the fate of each DNA strand through the meiotic process rather than depending on statistical analysis of the results of mass crosses. Explanations of the events in meioisis involving mismatch repair had been provided based on this new data by Harold Whitehouse (1963 ) and his student Robin Holliday (1964 ). These investigators argued that in the process of meiosis there was produced a region of DNA in which the two strands were not (necessarily) complementary but were derived from the two different parents, i.e. , the molecule itself was heterozygous. Completion of meiosis almost always involved conversion of the mismatch to a homozygous state by a process that Holliday identified as requiring enzymatic intervention and which consequently deviated from the expected Mendelian ratios. Holliday’s article was rejected by both Nature and GENETICS and was eventually published in Genetical Research ( Holloman 2014 )!
The (bio-) chemists seemed unable to respond. Genetics, as taught then (and unfortunately often still taught), started and ended with Mendel and his peas and the conventional ratios derived from so-called monohybrid and dihybrid crosses. The newer studies used ascomycete fungi ( e.g. , brewer’s yeast, Neurospora ) in which “all the products of a single meiotic event” could be analyzed. I believe that this phrase itself was mysterious to both biochemists and many (but not all) of the new molecular biologists. That the difference between a 4:4 ratio and a 5:3 ratio derived from a single meiotic event indicated the operation of a new biochemical pathway was not something easily accepted by those unfamiliar with these genetic systems.
As an example of the disconnect between genetics and biochemistry, consider the following: At a retreat, probably in the 1980s, for faculty and students in the Department of Molecular Genetics and Cell Biology at the University of Chicago, a student in Rochelle Esposito’s laboratory was explaining his research. He displayed Northern and Western blots without comment, knowing his audience would understand these, but stopped to explain the details of meiosis on the (probably correct) supposition that the details of this arcane biological process were probably unknown to these graduate students in biology, who would be unable to understand his research without such instruction.
Some prominent molecular biologists seemed quite willing to express their thoughts about genetics (or geneticists). Here, in 1968, is James Watson’s statement:
That was not to say that the geneticists themselves provided any intellectual help. You would have thought that with all their talk about genes they should worry about what they were. Yet almost none of them seemed to take seriously the evidence that genes were made of DNA. This fact was unnecessarily chemical. All that most of them wanted out of life was to set their students onto uninterpretable details of chromosome behavior or to give elegantly phrased, fuzzy-minded speculations over the wireless on topics like the role of genetics in this transitional age of changing values (Watson 1968, p. 74, my italics) ( Watson 1968 ).
Forty years later, consider this comparable view from a sketch of the history of molecular biology:
Geneticists seem to have been less pessimistic, perhaps because theirs was a subject that rejoiced in a multitude of essentially abstract words (dominant, recessive, epistatic and so on) — the kind of words that are designed to avoid the need for further thought ( Cairns 2008 ).
It is only fair to point out that the miscomprehension between these two fields is a two-way street. I can remember, as a graduate student, one cytologist being rather pleased that his subject could be handled in purely biological terms without reference to physics or chemistry. Further along, in the late 1960s, my graduate students insisted that we had to include an experiment with UV light in any article dealing with the repair of alkylation damage or the investigators studying DNA repair wouldn’t pay any attention!
It took almost 15 years to add biochemistry to Holliday’s 1964 suggestion, possibly because, as pointed out above, biochemists did not appreciate the problem or, more likely, because the tools were not available. An essential biochemical element was the discovery of DNA ligases: enzymes that joined broken DNA molecules. This finding was made in 1967 by at least four different laboratories ( Gefter et al. 1967 ; Olivera and Lehman 1967 ; Weiss and Richardson 1967 ; Zimmerman et al. 1967 ).
There was now good reason to suppose that recombination might actually be amenable to biochemical study. It was Matthew Meselson, using phage λ, who demonstrated that genetic recombination was accompanied by actual physical exchange of sections of DNA ( Meselson and Weigle 1961 ; Meselson 1964 ). Recombination therefore necessarily involved breaks in DNA and the models of recombination that were developed are similar to, and can be traced to, an understanding of how cells avoid the lethality associated with the production of double-strand breaks in DNA, a major consequence of ionizing radiation. I suggest that they derive from a recovery mechanism not involving excision called postreplication repair or replication repair first discovered by Rupp and Howard-Flanders (1968) and later shown to involve the product of the gene recA (see Smith and Wang 1989 ). When the DNA synthetic apparatus encounters a replication blocking lesion on one strand, it continues synthesis on the undamaged strand. Later, after synthesis of one double-stranded region, synthesis proceeds past the critical site on the damaged strand using the newly synthesized complementary strand as a template (see Higgins et al. 1976 for one way this might be done).
Haploid organisms such as bacteria require replication to provide such a template. Diploid organisms come equipped with a template for both strands as part of the homologous chromosome. The realization that an undamaged sister chromatid (or chromosome) could provide a template for the repair of both strands of DNA led to the general incorporation of repair concepts in the models of recombination that are the basis for current research ( Szostak et al. 1983 ).
The genetic data still required some sort of mismatch repair for a satisfactory interpretation of recombination (see above) but such data had an esoteric and nonconvincing air for biochemists who needed to know, among other things, how organisms recognized which strand needs correction. The discovery of methyl-directed mismatch repair in E. coli solved that problem ( Meselson and Radding 1975 ; Wagner and Meselson 1976 ) although we now know that these bacteria employ a sophisticated mechanism to discriminate between the old and newly synthesized DNA strand. The elegant work of the Modrich laboratory in particular then provided the specific mechanistic details ( Modrich 1986 ).
Recognition of the role of deficient mismatch repair in certain colon cancers ( Parsons et al. 1993 ) helped (in my opinion) to make the study of repair popular enough for Science magazine to decide that DNA repair enzymes were the 1994 “Molecule of the Year.” In 2000, a Cold Spring Harbor Symposium was devoted to Biological Responses to DNA Damage with the explanation on its website:
Discussion of the nature of mutations and genetic damage was pervasive at Cold Spring Harbor in the 1940s and 1950s due to the presence of Demerec, Max Delbrück, Salvador Luria, and Barbara McClintock and can be traced to the 1941 Symposium on “Genes and Chromosomes: Structure and Organization,” which occurred long before the double helix was revealed. We have come a long way in our understanding since then, and it was about time to correct the long absence of this topic from these Symposia ( Witkowski 2000 ).
This is a personal view of the early days of DNA repair studies. I have thought it useful to trace the development of our ideas about (mainly) excision repair to emphasize the importance of the double-stranded structure of DNA in the maintenance of genetic integrity. I have said nothing about the mutagenic SOS pathways studied so imaginatively by Evelyn Witkin and Miroslav Radman.
Without the existence of an active repair mechanism early in the history of life on earth and given the radiation flux in the prebiotic and early biotic environments, it would have been impossible for a molecule like DNA to have survived, let alone to have served as a stable repository of genetic information. Both single- and double-strand breaks in a polynucleotide chain would be necessarily lethal without repair, but the pathway(s) of restoration without introducing error is based on finding an undamaged homologous strand. It might therefore be argued that the double-stranded DNA structure was so successful in the evolution of living systems precisely because it provided a way to conserve genetic information as well as furnishing a mechanism for its copying.
It is hardly a surprise that students of a biological process should argue for the fundamental importance of the subject of their investigations. And it is therefore not surprising that many of us working on DNA repair should have felt that, although our studies seemed to us of vital interest, they were largely ignored by the molecular biologists we were trying to impress (see Friedberg 1997 ). Two questions can be posed: first, can anything of general interest can be learned from this history? Second, does the history itself actually matter?
This second question is easier to answer. The general “vote” on what is important in science has material consequences. Where should we put the money, what young person in what field should we hire? Once molecular biology was established as a scientific discipline in its own right, the opinions of the pioneers were hard to ignore. It is clear, as illustrated above, that many of them did think the mechanisms for correcting errors were mere details. Luckily, all of this happened in a period when support for science was (in retrospect) relatively lavish and the different fields were not in serious financial conflict, so the field of DNA repair could develop in part because of the link to cancer research and its funding provided by Cleaver’s discovery ( Cleaver 1968 ).
The history of the studies on mismatch repair illustrates another, possibly related, difficulty. Reading the studies on gene conversion and the articles of Whitehouse and Holliday with all the benefit of hindsight, it would seem that their connection to the newly described excision repair mechanisms would have been obvious. Yet it took at least a decade for mismatch repair as a separate process to be described. This must partly reflect the lag in discovering the enzymes that synthesized and glued together DNA chains. But it seems clear that both biochemists and (many of) the new molecular biologists were unable to take the genetic evidence seriously. I have copied some appropriate comments from James Watson, but the biochemists were equally parochial in their reluctance to accept a phenomenon as established without demonstrated mechanistic (enzymatic) detail ( Kornberg 2000 ). There was just no mutual understanding. It would be pleasant to believe that things are different today and that the emphasis on multi-disciplinary teams of investigators ensures that all avenues of potential interest will be explored. Perhaps.
Practical application of discovery follows unexpected paths. One of the most useful new biological technologies is the use of CRISPR-Cas9 and its modifications. The technology depends on the ability to make double-strand breaks at specific locations. What follows depends on the investigators making clever use of the various mechanisms for the repair of double-strand breaks along with mismatch repair. A Danish proverb attributed to Niels Bohr (and also to Yogi Berra) may be appropriate: “prediction is difficult, especially about the future.” Modesty about the relative importance of different approaches might be a desirable quality to cultivate at all times.
Errol Friedberg’s record of his many interviews with central participants ( Friedberg 1997 ) was a great help in preparation of this essay. I thank Phil Hanawalt for some insights into the exciting early days of nucleotide excision repair research.
Communicating editor: A. Wilkins
Auerbach C , Robson J M , 1947 The production of mutations by chemical substances. Proc. R. Soc. Edinb. Biol. 62 : 271 – 283 .
Google Scholar
Auerbach C , Robson J M , Carr J G , 1947 The chemical production of mutations. Science 105 : 243 – 247 . 10.1126/science.105.2723.243
Avery O T , Macleod C M , McCarty M , 1944 Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79 : 137 – 158 . 10.1084/jem.79.2.137
Bedford J S , Dewey W C , 2002 Radiation research society. 1952–2002. Historical and current highlights in radiation biology: has anything important been learned by irradiating cells? Radiat. Res. 158 : 251 – 291 . 10.1667/0033-7587(2002)158[0251:HACHIR]2.0.CO;2
Benzer S , Freese E , 1958 Induction of specific mutations with 5-Bromouracil. Proc. Natl. Acad. Sci. USA 44 : 112 – 119 . 10.1073/pnas.44.2.112
Beukers R , Berends W , 1960 Isolation and identification of the irradiation product of thymine. Biochim. Biophys. Acta 41 : 550 – 551 . 10.1016/0006-3002(60)90063-9
Bohr N , 1933 Light and life*. Nature 131 : 457 – 459 . 10.1038/131421a0
Boyce R P , Howard-Flanders P , 1964 Release of ultraviolet light-induced thymine dimers from DNA in E. Coli K-12. Proc. Natl. Acad. Sci. USA 51 : 293 – 300 . 10.1073/pnas.51.2.293
Cairns J , 2008 The foundations of molecular biology: a 50th anniversary. Curr. Biol. 18 : R234 – R236 . 10.1016/j.cub.2008.02.016
Chargaff E , 1950 Chemical specificity of nucleic acids and mechanism of their enzymatic degradation. Experientia 6 : 201 – 209 . 10.1007/BF02173653
Cleaver J E , 1968 Defective repair replication of DNA in xeroderma pigmentosum. Nature 218 : 652 – 656 . 10.1038/218652a0
Crick F , 1974 The double helix: a personal view. Nature 248 : 766 – 769 . 10.1038/248766a0
Crick F H , 1988 What Mad Pursuit . Basic Books , New York .
Google Preview
Daly M M , Allfrey V G , Mirsky A E , 1950 Purine and pyrimidine contents of some desoxypentose nucleic acids. J. Gen. Physiol. 33 : 497 – 510 . 10.1085/jgp.33.5.497
Fersht A R , Knill-Jones J W , Tsui W C , 1982 Kinetic basis of spontaneous mutation. Misinsertion frequencies, proofreading specificities and cost of proofreading by DNA polymerases of Escherichia coli . J. Mol. Biol. 156 : 37 – 51 . 10.1016/0022-2836(82)90457-0
Friedberg E , 1997 Correcting the Blueprint of Life: An Historical Account of the Discovery of DNA Repair Mechanisms . Cold Spring Harbor Laboratory Press , Cold Spring Harbor, NY .
Furst S S , Roll P M , Brown G , 1950 On the renewal of the purines of the desoxypentose and pentose nucleic acids. J. Biol. Chem. 183 : 251 – 266 .
Gefter M L , Becker A , Hurwitz J , 1967 The enzymatic repair of DNA. I. Formation of circular lambda-DNA. Proc. Natl. Acad. Sci. USA 58 : 240 – 247 . 10.1073/pnas.58.1.240
Hanawalt P C , Haynes R H , 1967 The repair of DNA. Sci. Am. 216 : 36 – 43 . 10.1038/scientificamerican0267-36
Haynes R H , Wolff S , Till J , 1965 Structural Defects in DNA and Their Repair in Microorganisms , edited by Haynes R H , Wolff S , Till J . Radiation Research , Chicago .
Hershey A D , Chase M , 1952 Independent functions of viral protein and nucleic acid in growth of bacteriophage. J. Gen. Physiol. 36 : 39 – 56 . 10.1085/jgp.36.1.39
Higgins N P , Kato K , Strauss B , 1976 A model for replication repair in mammalian cells. J. Mol. Biol. 101 : 417 – 425 . 10.1016/0022-2836(76)90156-X
Hill R F , 1958 A radiation-sensitive mutant of Escherichia coli. Biochim. Biophys. Acta 30 : 636 – 637 . 10.1016/0006-3002(58)90112-4
Holliday R , 1964 A mechanism for gene conversion in fungi. Genet. Res. 5 : 282 – 304 . 10.1017/S0016672300001233
Holloman B , 2014 Robin Holliday: 1932–2014. Cell 157 : 1001 – 1003 . 10.1016/j.cell.2014.05.005
Karran P , Lindahl T , Griffin B , 1979 Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature 280 : 76 – 77 . 10.1038/280076a0
Kelner A , 1949 Effect of visible light on the recovery of Streptomyces griseus conidia from ultra-violet irradiation injury. Proc. Natl. Acad. Sci. USA 35 : 73 – 79 . 10.1073/pnas.35.2.73
Kornberg A , 2000 Ten commandments: lessons from the enzymology of DNA replication. J. Bacteriol. 182 : 3613 – 3618 . 10.1128/JB.182.13.3613-3618.2000
Lahue R S , Modrich P , 1988 Methyl-directed DNA mismatch repair in Escherichia coli . Mutat. Res. 198 : 37 – 43 . 10.1016/0027-5107(88)90037-1
Lea D E , 1946 Actions of Radiations on Living Cells . Cambridge University Press , Cambridge, UK .
Lindahl T , 1974 An N -glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc. Natl. Acad. Sci. USA 71 : 3649 – 3653 . 10.1073/pnas.71.9.3649
Lindahl T , Nyberg B , 1972 Rate of depurination of native deoxyribonucleic acid. Biochemistry 11 : 3610 – 3618 . 10.1021/bi00769a018
Mazia D , 1952 Physiology of the cell nucleus , pp. 77 – 122 in Modern Trends in Physiology and Biochemistry , edited by Guzman-Barron E . Academic Press , New York .
McCulloch S D , Kunkel T A , 2008 The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 18 : 148 – 161 . 10.1038/cr.2008.4
Mellon I , Champe G N , 1996 Products of DNA mismatch repair genes mutS and mutL are required for transcription-coupled nucleotide-excision repair of the lactose operon in Escherichia coli. Proc. Natl. Acad. Sci. USA 93 : 1292 – 1297 . 10.1073/pnas.93.3.1292
Mellon I , Spivak G , Hanawalt P C , 1987 Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell 51 : 241 – 249 . 10.1016/0092-8674(87)90151-6
Meselson M , 1964 On the mechanism of genetic recombination between DNA molecules. J. Mol. Biol. 9 : 734 – 745 . 10.1016/S0022-2836(64)80178-9
Meselson M , Weigle J J , 1961 Chromosome breakage accompanying genetic recombination in bacteriophage. Proc. Natl. Acad. Sci. USA 47 : 857 – 868 . 10.1073/pnas.47.6.857
Meselson M S , Radding C M , 1975 A general model for genetic recombination. Proc. Natl. Acad. Sci. USA 72 : 358 – 361 . 10.1073/pnas.72.1.358
Modrich P , 1986 Mismatch correction. Basic Life Sci. 38 : 303 – 310 .
Moore J K , Haber J E , 1996 Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 16 : 2164 – 2173 . 10.1128/MCB.16.5.2164
Muller H J , 1927 Artificial transmutation of the gene. Science 66 : 84 – 87 . 10.1126/science.66.1699.84
Olivera B M , Lehman I R , 1967 Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc. Natl. Acad. Sci. USA 57 : 1426 – 1433 . 10.1073/pnas.57.5.1426
Parsons R , Li G M , Longley M J , Fang W H , Papadopoulos N et al. , 1993 Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell 75 : 1227 – 1236 . 10.1016/0092-8674(93)90331-J
Pettijohn D , Hanawalt P , 1964 Evidence for repair-replication of ultraviolet damaged DNA in bacteria. J. Mol. Biol. 9 : 395 – 410 . 10.1016/S0022-2836(64)80216-3
Pettijohn D E , Hanawalt P C , 1963 Deoxyribonucleic acid replication in bacteria following ultraviolet irradiation. Biochim. Biophys. Acta 72 : 127 – 129 . 10.1016/0926-6550(63)90324-4
Polosina Y Y , Cupples C G , 2010 MutL: conducting the cell’s response to mismatched and misaligned DNA. BioEssays 32 : 51 – 59 . 10.1002/bies.200900089
Reiter H , Strauss B , 1965 Repair of damage induced by a monofunctional alkylating agent in a transformable, ultraviolet-sensitive strain of Bacillus subtilis . J. Mol. Biol. 14 : 179 – 194 . 10.1016/S0022-2836(65)80239-X
Rupp W D , Howard-Flanders P , 1968 Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J. Mol. Biol. 31 : 291 – 304 . 10.1016/0022-2836(68)90445-2
Sagan C , 1961 On the origin and planetary distribution of life. Radiat. Res. 15 : 174 – 192 . 10.2307/3571249
Schoenheimer R , 1942 The Dynamic State of Body Constituents . Harvard University Press , Cambridge, MA .
Schrödinger E , 1945 What Is Life? The Physical Aspect of the Living Cell The Macmillan Company , New York .
Setlow R B , Carrier W L , 1964 The disappearance of thymine dimers from DNA: an error-correcting mechanism. Proc. Natl. Acad. Sci. USA 51 : 226 – 231 . 10.1073/pnas.51.2.226
Smith K C , Wang T C , 1989 recA-dependent DNA repair processes. BioEssays 10 : 12 – 16 . 10.1002/bies.950100104
Sobels F H (Editor), 1963 Repair from Genetic Radiation Damage . The MacMillan Company , New York .
Stadler L J , 1928 a Genetic effects of X-rays in maize. Proc. Natl. Acad. Sci. USA 14 : 69 – 75 . 10.1073/pnas.14.1.69
Stadler L J , 1928 b Mutations in barley induced by X rays and radium. Science 68 : 186 – 187 . 10.1126/science.68.1756.186
Stone W S , Wyss O , Haas F , 1947 The production of mutations in Staphylococcus aureus by irradiation of the substrate. Proc. Natl. Acad. Sci. USA 33 : 59 – 66 . 10.1073/pnas.33.3.59
Strauss B S , 1963 Recovery of deoxyribonucleic acid from the effects of alkylation. J. Gen. Microbiol. 30 : 89 – 103 . 10.1099/00221287-30-1-89
Strauss B S , 2017 A physicist’s quest in biology: Max Delbrück and “complementarity.” Genetics 206 : 641 – 650 . 10.1534/genetics.117.201517
Szostak J W , Orr-Weaver T L , Rothstein R J , Stahl F W , 1983 The double-strand-break repair model for recombination. Cell 33 : 25 – 35 . 10.1016/0092-8674(83)90331-8
Timofeeff-Ressovsky N W , Zimmer K G , Delbrück M , 1935 Über die Natur der Genmutation und der Genstruktur. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Math.-. Phys. Kl Fachgruppe 6 : 190 – 245 .
Wagner R Jr. , Meselson M , 1976 Repair tracts in mismatched DNA heteroduplexes. Proc. Natl. Acad. Sci. USA 73 : 4135 – 4139 . 10.1073/pnas.73.11.4135
Watson J D , 1968 The Double Helix . A Personal Account of the Discovery of the Structure of DNA . Atheneum , New York .
Watson J D , Crick F H , 1953 a Genetical implications of the structure of deoxyribonucleic acid. Nature 171 : 964 – 967 . 10.1038/171964b0
Watson J D , Crick F H , 1953 b Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 171 : 737 – 738 . 10.1038/171737a0
Weiss B , Richardson C C , 1967 Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc. Natl. Acad. Sci. USA 57 : 1021 – 1028 . 10.1073/pnas.57.4.1021
Whitehouse H L , 1963 A theory of crossing-over by means of hybrid deoxyribonucleic acid. Nature 199 : 1034 – 1040 . 10.1038/1991034a0
Witkin E M , 1956 Time, temperature, and protein synthesis: a study of ultraviolet-induced mutation in bacteria. Cold Spring Harb. Symp. Quant. Biol. 21 : 123 – 140 . 10.1101/SQB.1956.021.01.011
Witkowski, J. A., 2000 Introduction to Biological Responses to DNA Damage , Vol. LXV . Cold Spring Harbor Symposia on Quantitative Biology. Available at: http://symposium.cshlp.org/site/misc/topic65.xhtml .
Wood R D , Mitchell M , Lindahl T , 2005 Human DNA repair genes, 2005. Mutat. Res. 577 : 275 – 283 . 10.1016/j.mrfmmm.2005.03.007
Wulff D L , Fraenkel G , 1961 On the nature of thymine photo-product. Biochim. Biophys. Acta 51 : 332 – 339 . 10.1016/0006-3002(61)90174-3
Wulff D L , Rupert C S , 1962 Disappearance of thymine photodimer in ultraviolet irradiated DNA upon treatment with a photoreactivating enzyme from baker’s yeast. Biochem. Biophys. Res. Commun. 7 : 237 – 240 . 10.1016/0006-291X(62)90181-X
Zimmerman S B , Little J W , Oshinsky C K , Gellert M , 1967 Enzymatic joining of DNA strands: a novel reaction of diphosphopyridine nucleotide. Proc. Natl. Acad. Sci. USA 57 : 1841 – 1848 . 10.1073/pnas.57.6.1841
| Month: | Total Views: |
|---|---|
| January 2021 | 2 |
| February 2021 | 13 |
| March 2021 | 10 |
| April 2021 | 13 |
| May 2021 | 11 |
| June 2021 | 11 |
| July 2021 | 11 |
| August 2021 | 4 |
| September 2021 | 13 |
| October 2021 | 25 |
| November 2021 | 17 |
| December 2021 | 67 |
| January 2022 | 359 |
| February 2022 | 303 |
| March 2022 | 339 |
| April 2022 | 287 |
| May 2022 | 235 |
| June 2022 | 243 |
| July 2022 | 145 |
| August 2022 | 142 |
| September 2022 | 340 |
| October 2022 | 498 |
| November 2022 | 381 |
| December 2022 | 232 |
| January 2023 | 331 |
| February 2023 | 259 |
| March 2023 | 329 |
| April 2023 | 217 |
| May 2023 | 156 |
| June 2023 | 129 |
| July 2023 | 138 |
| August 2023 | 144 |
| September 2023 | 182 |
| October 2023 | 182 |
| November 2023 | 132 |
| December 2023 | 126 |
| January 2024 | 142 |
| February 2024 | 123 |
| March 2024 | 100 |
| April 2024 | 54 |
| May 2024 | 97 |
| June 2024 | 45 |
| July 2024 | 39 |
| August 2024 | 34 |
| September 2024 | 68 |
Email alerts
Companion article.
- ISSUE HIGHLIGHTS
Citing articles via
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1943-2631
- Copyright © 2024 Genetics Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
https://lessonsonscience.com
essays on science for the common good
Part XX: DNA or Protein; The Great Debate
The story of the true carrier of genetic information from generation to generation is an interesting tale which followed a winding path from the late 1800’s up to 1953 and even that historic discovery, while one of the most sought after and important discoveries of human kind, was really just a new chapter in the book of life as we know it that continues today and undoubtable will as long as human civilization exists.
The story really begins in 1869 when a German physician Fredrich Miescher discovered a white, sugary, slightly acidic substance that contained phosphorus. He named it “nuclein” based on its presence only in the nucleus of cells. Fast forward to 1914the year that Prince Ferdinand of Austria was assassinated thus beginning “The Great War” (World War I) Robert Fuelgen , another German, found that DNA was strongly attracted to fuchsin, a red dye. Just a small step but helpful one. Later it was discovered that DNA was present in all cells and was associated with the chromosomes which had only recently been discovered. Let’s take a small “aside” now and delve a little further into the world of the chromosome. Oscar Hertwig, yet another German (an embryologist) observed sea urchin fertilization and realized that only one sperm cell was necessary to fertilize an egg and further noted that when the sperm cell penetrated egg the nuclei from the sperm and egg fuse. This established that the nucleus, or something in it, is the carrier of genetic information from generation to generation, another small step. At about the same time, Walther Flemming observed the “dance of the chromosomes” during cell reproduction, “steps” of mitosis (cell division), and the events of each step. Of course, in reality, the process is continuous and people devised the “steps” to understand the process better. To learn more including the phases such as prophase, metaphase, anaphase, telophase, etc., simple Google “mitosis” or “cell division” or consult any high school biology textbook. In one experiment Flemming removed the nucleus of an ameba (amoeba for our British friends) and watched the cell die.
P.A. Levene , a biochemist proved in the 1920’s that DNA was composed of a 5- carbon sugar (a pentose); a phosphate group, and four nitrogenous bases, adenine and guanine (purines) and thymine and cytosine (pyrimidines). He concluded that each nitrogenous base is connected to a sugar molecule which is attached to a phosphate group which makes up a nucleotide. (See essay IX Majors)
In 1928, Frederich Griffith , an English bacteriologist public health official was trying to develop a vaccine against S treptococcus pneumonia which causes a form of pneumonia. This bacterium comes in two forms, one a virulent (disease causing) form with a polysaccharide (simple sugar capsule and a nonvirulent (harmless) none capsulated form. Griffith wanted to know if injections of heat killed virulent pneumoiae could be used to immunize against pneumonia. At one point he injected mice simultaneously with heat kicked virulent bacteria and living non-virulent bacteria expecting the ice to live but all the mice died—a complete surprise. Autopsies on the mice revealed that their bodies were filled with living encapsulated virulent bacteria. Years later it was shown that extracts from heat killed virulent bacteria when added to harmless bacteria could transform them into harmful bacteria complete with protective capsules. Thus an extremely important phenomenon called transformation was discovered and the yet unknown substance responsible for this transformation was called a transforming factor. (“Factor” is still used to refer to an unknown substance). Later this transforming factor was identified as—you guessed it—DNA. When I presented this in class I usually describe the heat killed bacteria as never-say-die KILLER BACTERIA for dramatic effect. It was an American scientist, O. T. Avery that identified DNA as the transforming factor.
At this point it must seem like DNA was the overwhelming candidate for carrying the genetic information from generation to generation—but, hold on, don’t count the other contender out yet. Max Delbruck and Salvador Luria two scientists who emigrated from Europe during the intellectual mass exodus of the 1930’s along with Albert Einstein,
And other mathematicians and physicists prior to the Third Reich takeover of 1939performed some Nobel Prize experiments in 1940 with a special group of viruses called bacteriophages or simply phages because they infect bacteria. Yes, even bacteria have enemies. The particular viruses of interest in their research ae called coliphage and they attach Escherichia coli . They were numbered T1 through t7 (“T” means “type”). In a personal note here I very recently inoculated E. coli bacteria with T4 coliphage in my home lab attempting to do a T4 assay, See the photo below.
Skipping the details of the experiments, chemical analysis of fragments of phages after infection revealed what we all know now – that viruses are composed of just two organic compounds, a DNA core and a protein coat. Later RNA was also discovered in the core but if so DNA is absent (see essay IX). So now the great debate ha had been brewing for years suddenly intensified. What carried the all-important genetic code, DNA or protein? Scientists were divided into two camps. The protein backers had a very simple philosophy: DNA is composed of just 4 nucleotides but proteins are composed of 20 amino acids (see essay IX). They reasoned that like an alphabet composed of 20 letters could spell more words than one with 4 letters, so proteins could account for greater genetic variability (diversity).
The stage was now set for the most compelling evidence yet. In 1952 two researchers Alfred Hershey and Martha Chase performed a brilliant set of experiments based on two simple differences in DNA and proteins. But first I always made a point of emphasizing that like Rosiland Franklin, Martha Chase was a pioneer in that here was a woman that rose to prominence in a field dominated by men and they could do the same much to their delight. OK, another personal aside here.
A few years ago (2013), I ran into a former student in a local Kohl’s Department Store. She also became a lab assistant and a very good one who earned her degree at the University of Iowa and now was doing scientific research at Marquette University. She told me that she got her start after being my student and lab assistant. Talk about delight! I was on cloud 9 at a time I was going through a long, tough, tough time medically. Then she topped it off by saying that I looked the same as I did when I was her teacher in the mid ‘80’s. This was at a time when I looked terrible and felt much worse.
Back to Hershey and Chase: DNA as we know, contain phosphorus but proteins don’t. Proteins contain sulfur but DNA doesn’t. They prepared two groups of viruses, one which was labeled with radioactive phosphorus ( 32 P)and one labeled with radioactive sulfur ( 35 S) and inoculated then into an E. coli host with the appropriate radioactive isotope. Again skipping some of the details one culture of bacteria was infected with 32 P and another infected with 35 Sphage and later were tested for radioactivity. Their brilliantly conceived experiments revealed that the 35 S phages had remained outside the bacterial cells but the 32 P (DNA) had entered the cells, infected the bacteria and produced new viruses. The great debate was essentially over; DNA was proclaimed the winner.
Erwin Chargaff of Columbia University analyzed the purine and pyrimidines content from many different species of organisms. Here is a sample of his results showing percentages of the four bases.
Purines Pyrimidines source adenine guanine cytosine thymine
Human 30.4% 19.6% 19.9% 30.1% Ox 29.0 21.2 21.1 28.7
Wheat germ 28.1 21.8 22.7 27.4 after examining the data what can you conclude? Answer as usual at the end of the essay.
It is almost anticlimactic to now talk about James Watson and Francis Crick’s famous discovery in 1953. Watson, a former Whiz Kid from Chicago was going to become an ornithologist but thankfully changed careers. Francis Crick, a trained physicist were an unlikely fil for one of the most important discoveries in the history of science. Rather than swell on their work which would extend this very long essay much longer I would recommend reading a form of Watson’s famous book “ The Double Helix ” and again read the last p [art of essay IX (majors). I have read his book at least three times and learned more each time. It’s written so that most ordinary people can understand it with just a little science background. If you can/t or don’t want to read the book (a short one in terms of book length, I would suggest Googling “The Double Helix”.
I briefly described protein synthasis in essay IX but now direct you to a “code of life chart”. One of the main mysteries to be delved was “breaking the code”, that is, to learn how
DNA directs:
- its own replication
- protein synthesis
Now that we know DNA directs protein synthesis let’s look in more detail at the overall process. According to “central dogma”, a term that Crick himself coined, during protein synthesis the double stranded DNA molecule splits down the middle (hydrogen bonds break releasing emery) and each side serves as a template for a strand of messenger RNA (mRNA with the cytocine of DNA coding for a guanine of mRNA and adenine of DNA coding for uracil of mRNA. Remember that thymine in DNA is replaced by uracil in RNA. However, thymine of DNA still codes for adenine of RNA. This whole process is called transcription , a process that occurs in the nucleus. The single stranded mRNA leaves the nucleus and pairs with a transfer RNA (tRNA) which contains an amino acid (the building block of proteins) on one end and an attachment on the other end to enter a ribosome (ribosomal RNA or rRNA). As stated in an earlier essay, it’s as if the mRNA molecule says to the tRNA, “let’ meet at the ribosome (more correctly let’s go to the ribosome) and make a protein”, a process called translation . The long strand of mRNA can be thought of as individual units of codons (three nitrogenous bases per codon) that like transcription from DNA, pairs with the corresponding anticodon on the tRNA molecule. That is, a u from mRNA pairs with an a of tRNA, c with g, g with c, etc. Remember though an a pairs with a u of tRNA. Why? The individual tRNA subunits join together inside the ribosome (rRNA) and exit out in long polypeptide chains which when long enough and exhibit secondary structures and perhaps tertiary and quaternary structures are called proteins. But remember, and this is huge, it all began with DNA.
Let’s pause for a moment and reflect on some important principles.
- DNA is composed of 4 nucleotides, a four letter alphabet (the four nitrogenous bases)
- There are 20 amino acids commonly found in living organisms
- Codons (and, therefore, anticodons) occur in triplets
Now the question arises of why groups of 3 bases in a codon? Why not 1 or 2?
- Individual codons could code for only 4 amino acids. (4 1 ) 4 bases raised to fist power if just one individual base
- Pairs of codons could produce only 16 amino acids (4 2 )
- Triplets could produce 64 amino acids (4 3 ) 4x4x4= 64 which is more than enough for the 20 amino acids In fact, this means that there must be more than one codon that can code for each amino acid, right? Right.
Now we are ready for the ultimate task and the climax of this entire essay and perhaps all of my essays except for perhaps those on climate change. Identifying the correct structure of the DNA molecule is one thing but to apply it to genetics and understand how I works in specifying the production of the thousands of chemical compounds and chemical reaction each with its own enzyme in our bodies is a totally different set of circumstances. But let’ proceed. Here is the “code of life” chart that summarizes (I didn’t say explains) all of the previous sentences.

The letters along the left side, top, and right side represent the 4 bases. You know their names by now. The letters (in triplet) represent the codons and the abbreviation of individual amino acids. To select amino acids:
- choose a letter on the left side
- choose a letter on top
- choose a letter on the right side
- write them down and identify the amino acid using the listing below using the abbreviations.

Credit to: RearchGate
I’ll do two examples for you.
Q. Find the code(s) for cysteine (cys)
A. UGU, UGC
Q. Find codes for serine (ser)
A. AGU, AGC
Let’s reverse the process.
Q. What does the codon CCU code for?
A. Arginine
Now for a real problem.
Find the code for
Alanine(ala) plus glycine(gly) plus valine (val)
Feeling pretty good? Try another code for them.

Published by Larry Baumer
I graduated from Northern Illinois University in 1966 with a Bachelor of Science degree in Education and earned a Master of Science degree in Education also from NIU in 1973. I taught in the Harlem School District (5 years), a Chicago suburb (1 year), and the Rockford, IL School District for 27 years (26 at East High School). I culminated my teaching career at Kishwaukee College (8 years) Two important events occurred in 1988: I married my wife Angie and I received a summer teacher's research fellowship through the University of Illinois School of Medicine at Rockford. My primary responsibility was light microscopy and Scanning electron miscroscopy of rabbit renal arteries (effect of high cholesterol diet). For 14 years I was a citizen scientist for the Illinois Department of Natural Resources in their RiverWatch program (monitoring water quality) My hobbies and activities include gardening, golfing, bowling, downhill and cross country skiing, photography, including photomicroscopy and time lapse photography, spending time with my wife and our dog, and in the winter playing around in my small home biology & chemistry lab. Beyond what I have written in past profiles, in the early 1980’s I was an EMT with the Boone Volunteer Ambulance & Rescue Squad (BVARS) which fit in nicely with my science training and teaching. I also enjoy public speaking and made frequent scholarship presentations to graduating seniors and outstanding middle school students through the former Belvidere Y’ Men’s Club. I also made power point presentations of the RiverWatch program. But I most enjoyed making presentations at my high school reunions. Thanks guys for allowing me to do this. I have submitted four poems and one short story (bittersweet) to the editors of Chicken Soup for the Soul of a previous beloved dog but I am still waiting…. View more posts
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Human Enhancement
The scientific and ethical dimensions of striving for perfection.
H uman enhancement is at least as old as human civilization. People have been trying to enhance their physical and mental capabilities for thousands of years, sometimes successfully – and sometimes with inconclusive, comic and even tragic results.
Up to this point in history, however, most biomedical interventions, whether successful or not, have attempted to restore something perceived to be deficient, such as vision, hearing or mobility. Even when these interventions have tried to improve on nature – say with anabolic steroids to stimulate muscle growth or drugs such as Ritalin to sharpen focus – the results have tended to be relatively modest and incremental.

But thanks to recent scientific developments in areas such as biotechnology, information technology and nanotechnology, humanity may be on the cusp of an enhancement revolution. In the next two or three decades, people may have the option to change themselves and their children in ways that, up to now, have existed largely in the minds of science fiction writers and creators of comic book superheroes.
Both advocates for and opponents of human enhancement spin a number of possible scenarios. Some talk about what might be called “humanity plus” – people who are still recognizably human, but much smarter, stronger and healthier. Others speak of “post-humanity,” and predict that dramatic advances in genetic engineering and machine technology may ultimately allow people to become conscious machines – not recognizably human, at least on the outside.
This enhancement revolution, if and when it comes, may well be prompted by ongoing efforts to aid people with disabilities and heal the sick. Indeed, science is already making rapid progress in new restorative and therapeutic technologies that could, in theory, have implications for human enhancement.
It seems that each week or so, the headlines herald a new medical or scientific breakthrough. In the last few years, for instance, researchers have implanted artificial retinas to give blind patients partial sight . Other scientists successfully linked a paralyzed man’s brain to a computer chip , which helped restore partial movement of previously non-responsive limbs. Still others have created synthetic blood substitutes , which could soon be used in human patients.
One of the most important developments in recent years involves a new gene-splicing technique called “clustered regularly interspaced short palindromic repeats.” Known by its acronym, CRISPR , this new method greatly improves scientists’ ability to accurately and efficiently “edit” the human genome, in both embryos and adults.
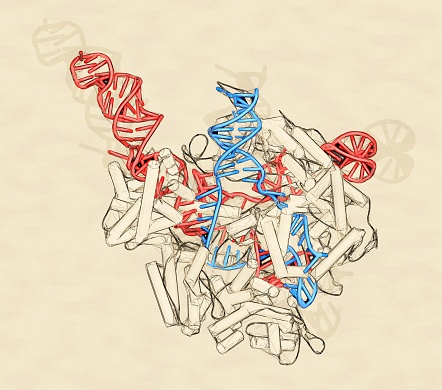
To those who support human enhancement, many of whom call themselves transhumanists, technological breakthroughs like these are springboards not only to healing people but to changing and improving humanity. Up to this point, they say, humans have largely worked to control and shape their exterior environments because they were powerless to do more. But transhumanists predict that a convergence of new technologies will soon allow people to control and fundamentally change their bodies and minds. Instead of leaving a person’s physical well-being to the vagaries of nature, supporters of these technologies contend, science will allow us to take control of our species’ development, making ourselves and future generations stronger, smarter, healthier and happier.
The science that underpins transhumanist hopes is impressive, but there is no guarantee that researchers will create the means to make super-smart or super-strong people. Questions remain about the feasibility of radically changing human physiology, in part because scientists do not yet completely understand our bodies and minds. For instance, researchers still do not fully comprehend how people age or fully understand the source of human consciousness.
There also is significant philosophical, ethical and religious opposition to transhumanism. Many thinkers from different disciplines and faith traditions worry that radical changes will lead to people who are no longer either physically or psychologically human.
Even minor enhancements, critics say, may end up doing more harm than good. For instance, they contend, those with enhancements may lack empathy and compassion for those who have not chosen or cannot afford these new technologies. Indeed, they say, transhumanism could very well create an even wider gap between the haves and have-nots and lead to new kinds of exploitation or even slavery.
Given that the science is still at a somewhat early stage, there has been little public discussion about the possible impacts of human enhancement on a practical level. But a new survey by Pew Research Center suggests wariness in the U.S. public about these emerging technologies. For example, 68% of Americans say they would be “very” or “somewhat” worried about using gene editing on healthy babies to reduce the infants’ risk of serious diseases or medical conditions. And a majority of U.S. adults (66%) say they would “definitely” or “probably” not want to get a brain chip implant to improve their ability to process information.
And yet, perhaps ironically, enhancement continues to captivate the popular imagination. Many of the top-grossing films in recent years in the United States and around the world have centered on superheroes with extraordinary abilities, such as the X-Men, Captain America, Spiderman, the Incredible Hulk and Iron Man. Such films explore the promise and pitfalls of exceeding natural human limits.
HUMAN ENHANCEMENT IN POPULAR CULTURE
[flipcards images=”https://www.pewresearch.org/wp-content/uploads/sites/9/2016/07/PS_2016.07.26_Human-Enhancement-Essay_Daedalus-250px.jpg, https://www.pewresearch.org/wp-content/uploads/sites/9/2016/07/PS_2016.07.26_Human-Enhancement-Essay_Frankenstein-250px.jpg, https://www.pewresearch.org/wp-content/uploads/sites/9/2016/07/PS_2016.07.26_Human-Enhancement-Essay_Gattaca-250px.jpg, https://www.pewresearch.org/wp-content/uploads/sites/9/2016/07/PS_2016.07.26_Human-Enhancement-Essay_Cap-250px.jpg” backs=”In the Greek myth, Daedalus fashioned wax and feather wings so that he and son Icarus could fly. But Icarus fell to his death because he flew too close to the sun, melting the wax., In Mary Shelley’s “Frankenstein” a scientist creates a new man only to ultimately die while trying to destroy his creation., The film Gattaca takes place in a future where non-genetically enhanced humans are considered “invalid.”, In the movies and comics, Captain America is a genetically-enhanced superhuman created to fight in America’s wars.”]
Not only is enhancement unquestionably part of today’s cultural zeitgeist, questions about humanity’s quest to move beyond natural limits go back to our earliest myths and stories. The ancient Greeks told of Prometheus, who stole fire from the gods, and Daedalus, the skilled craftsman, who made wings for himself and his son, Icarus. In the opening chapters of Genesis, the Hebrew Bible depicts a successful incident of human enhancement, when Adam and Eve ate the fruit from the tree of the knowledge of good and evil because the Serpent told them it would make them “like God.”
Of course, while Adam and Eve gained a new awareness and self-understanding, their actions also led to their expulsion from paradise and entry into a much harder world full of pain, shame and toil. This theme – that hidden dangers may lurk in something ostensibly good – runs through many literary accounts of enhancement. In Mary Shelley’s “Frankenstein” (1818), for instance, a scientist creates a new man, only to eventually die while trying to destroy his creation.
Whether these fears surrounding human enhancement are real or unfounded is a question already being debated by ethicists, scientists, theologians and others. This report looks at that debate, particularly in light of the diverse religious traditions represented in the United States. First, though, the report explains some of the scientific developments that might form the basis of an enhancement revolution.
[chapter title=”Where does the science stand?” background_image=”16058″]
O n Feb. 25, 2014, President Barack Obama met with Army officials and engineers at the Pentagon to discuss plans to create a new super armor that would make soldiers much more dangerous and harder to kill. The president joked that “we’re building ‘Iron Man,’” but Obama’s jest contained more than a kernel of truth: The exoskeleton, called the Tactical Assault Light Operator Suit (TALOS), does look vaguely like the fictional Tony Stark’s famous Iron Man suit. The first prototypes already are being built, and if all goes as planned, American soldiers may soon be much stronger and largely impervious to bullets.
A little more than a year later and an ocean away, scientists with the United Kingdom’s National Health Service (NHS) announced that by 2017, they plan to begin giving human subjects synthetic or artificial blood . If the NHS moves ahead with its plans, it would be the first time people receive blood created in a lab. While the ultimate aim of the effort is to stem blood shortages, especially for rare blood types, the success of synthetic blood could lay the foundation for a blood substitute that could be engineered to carry more oxygen or better fight infections.
In April 2016, scientists from the Battelle Memorial Institute in Columbus, Ohio, revealed that they had implanted a chip in the brain of a quadriplegic man. The chip can send signals to a sleeve around the man’s arm, allowing him to pick up a glass of water, swipe a credit card and even play the video game Guitar Hero .
Roughly around the same time, Chinese researchers announced they had attempted to genetically alter 213 embryos to make them HIV resistant. Only four of the embryos were successfully changed and all were ultimately destroyed. Moreover, the scientists from the Guangzhou Medical University who did the work said its purpose was solely to test the feasibility of embryo gene editing, rather than to regularly begin altering embryos. Still, Robert Sparrow of Australia’s Monash University Centre for Human Bioethics said that while editing embryos to prevent HIV has an obvious therapeutic purpose, the experiment more broadly would lead to other things. “Its most plausible use, and most likely use, is the technology of human enhancement,” he said, according to the South China Morning Post .
As these examples show, many of the fantastic technologies that until recently were confined to science fiction have already arrived, at least in their early forms. “We are no longer living in a time when we can say we either want to enhance or we don’t,” says Nicholas Agar , a professor of ethics at Victoria University in Wellington, New Zealand, and author of the book “Humanity’s End: Why We Should Reject Radical Enhancement.” “We are already living in an age of enhancement.”
The road to TALOS, brain chips and synthetic blood has been a long one that has included many stops along the way. Many of these advances come from a convergence of more than one type of technology – from genetics and robotics to nanotechnology and information technology. These technologies are “intermingling and feeding on one another, and they are collectively creating a curve of change unlike anything we humans have ever seen,” journalist Joel Garreau writes in his book “ Radical Evolution : The Promise and Peril of Enhancing Our Minds, Our Bodies – and What It Means to Be Human.”
The combination of information technology and nanotechnology offers the prospect of machines that are, to quote the title of Robert Bryce’s recent book on innovation, “Smaller Faster Lighter Denser Cheaper.” And as some futurists such as Ray Kurzweil argue, these developments will occur at an accelerated rate as technologies build on each other. “An analysis of the history of technology shows that technological change is exponential, contrary to the common-sense ‘intuitive linear’ view,” writes Kurzweil , an American computer scientist and inventor whose work has led to the development of everything from checkout scanners at supermarkets to text-reading machines for the blind. “So we won’t experience 100 years of progress in the 21st century – it will be more like 20,000 years of progress (at today’s rate).”
[icon_headline headline=”GENETIC EDITING AND ENGINEERING” image=”16088″ align=”aligntop”]
In the field of biotechnology, a big milestone occurred in 1953, when American biologist James Watson and British physicist Francis Crick discovered the molecular structure of DNA – the famed double helix – that is the genetic blueprint for life. Almost 50 years later, in 2003, two international teams of researchers led by American biologists Francis Collins and Craig Venter succeeded in decoding and reading that blueprint by identifying all of the chemical base pairs that make up human DNA.
Finding the blueprint for life, and successfully decoding and reading it, has given researchers an opportunity to alter human physiology at its most fundamental level. Manipulating this genetic code – a process known as genetic engineering – could allow scientists to produce people with stronger muscles, harder bones and faster brains. Theoretically, it also could create people with gills or webbed hands and feet or even wings – and, as Garreau points out in his book, could lead to “an even greater variety of breeds of humans than there is of dogs.”

In recent years, the prospect of advanced genetic engineering has become much more real, largely due to two developments. First, inexpensive and sophisticated gene mapping technology has given scientists an increasingly more sophisticated understanding of the human genome.
[than existing methods]
CRISPR is already dramatically expanding the realm of what is possible in the field of genetic engineering. Indeed, on June 21, 2016, the U.S. government announced that it had approved the first human trials using CRISPR, in this case to strengthen the cancer-fighting properties of the immune systems of patients suffering from melanoma and other deadly cancers. “CRISPR’s power and versatility have opened up new and wide-ranging possibilities across biology and medicine,” says Jennifer Doudna , a researcher at the University of California at Berkeley and a co-inventor of CRISPR.
According to Doudna and others, CRISPR could provide new treatments or even cures to some of today’s most feared diseases – not only cancer, but Alzheimer’s disease, Parkinson’s disease and others.
Jennifer Doudna, UC Berkeley
CRISPR’s power and versatility has opened up new and wide-ranging possibilities across biology and medicine.
[/pullquote]
An even more intriguing possibility involves making genetic changes at the embryonic stage, also known as germline editing. The logic is simple: alter the gene lines in an embryo’s eight or 16 cell stage (to, say, eliminate the gene for Tay-Sachs disease) and that change will occur in each of the resulting person’s trillions of cells – not to mention in the cells of their descendants. When combined with researchers’ growing understanding of the genetic links to various diseases, CRISPR could conceivably help eliminate a host of maladies in people before they are born.
But many of the same scientists who have hailed CRISPR’s promise, including Doudna, also have warned of its potential dangers. At a National Academy of Sciences conference in Washington, D.C., in December 2015, she and about 500 researchers, ethicists and others urged the scientific community to hold off editing embryos for now, arguing that we do not yet know enough to safely make changes that can be passed down to future generations.
Those at the conference also raised another concern: the idea of using the new technologies to edit embryos for non-therapeutic purposes. Under this scenario, parents could choose a variety of options for their unborn children, including everything from cosmetic traits, such as hair or eye color, to endowing their offspring with greater intellectual or athletic ability. Some transhumanists see a huge upside to making changes at the embryonic level. “This may be the area where serious enhancement first becomes possible, because it’s easier to do many things at the embryonic stage than in adults using traditional drugs or machine implants,” says Nick Bostrom, director of the Future of Humanity Institute , a think tank at Oxford University that focuses on “big picture questions about humanity and its prospects.”
But in the minds of many philosophers, theologians and others, the idea of “designer children” veers too close to eugenics – the 19th- and early 20th-century philosophical movement to breed better people. Eugenics ultimately inspired forced sterilization laws in a number of countries (including the U.S.) and then, most notoriously, helped provide some of the intellectual framework for Nazi Germany’s murder of millions in the name of promoting racial purity.
There also may be practical obstacles. Some worry that there could be unintended consequences, in part because our understanding of the genome, while growing, is not even close to complete. Writing in Time magazine , Venter, who helped lead the first successful effort to sequence the human genome, warns that “we have little or no knowledge of how (with a few exceptions) changing the genetic code will effect development and the subtlety associated with the tremendous array of human traits.” Venter adds: “Genes and proteins rarely have a single function in the genome and we know of many cases in experimental animals where changing a ‘known function’ of a gene results in developmental surprises.”
[icon_headline headline=”A BETTER BRAIN?” image=”16097″ align=”aligntop”]
For many transhumanists, expanding our capacities begins with the organ that most sets humans apart from other animals: the brain. Right now, cognitive enhancement largely involves drugs that were developed and are prescribed to treat certain brain-related conditions, such as Ritalin for attention deficit disorder or modafinil for narcolepsy. These and other medications have been shown in lab tests to help sharpen focus and improve memory.
But while modafinil and other drugs are now sometimes used (off label) to improve cognition, particularly among test-cramming students and overwhelmed office workers, the improvements in focus and memory are relatively modest. Moreover, many transhumanists and others predict that while new drugs (say, a specifically designed, IQ-boosting “smart pill”) or genetic engineering could result in substantially enhanced brain function, the straightest and shortest line to dramatically augmenting cognition probably involves computers and information technology.
As with biotechnology, information technology’s story is littered with important milestones and markers, such as the development of the transistor by three American scientists at Bell Labs in 1947. Transistors are the electronic signal switches that gave rise to modern computers. By shrinking the electronic components to microscopic size, researchers have been able to build ever smaller, more powerful and cheaper computers. As a result, today’s iPhone has more than 250,000 times more data storage capacity than the guidance computer installed on the Apollo 11 spacecraft that took astronauts to the moon.
One of the reasons the iPhone is so powerful and capable is that it uses nanotechnology, which involves “ the ability to see and to control individual atoms and molecules .” Nanotechnology has been used to create substances and materials found in thousands of products, including items much less complex than an iPhone, such as clothing and cosmetics.
Advances in computing and nanotechnology have already resulted in the creation of tiny computers that can interface with our brains. This development is not as far-fetched as it may sound, since both the brain and computers use electricity to operate and communicate. These early and primitive brain-machine interfaces have been used for therapeutic purposes, to help restore some mobility to those with paralysis (as in the example involving the quadriplegic man) and to give partial sight to people with certain kinds of blindness. In the future, scientists say, brain-machine interfaces will do everything from helping stroke victims regain speech and mobility to successfully bringing people out of deep comas.
Right now, most scientists working in the brain-machine-interface field say they are solely focused on healing, rather than enhancing. “I’ve talked to hundreds of people doing this research, and right now everyone is wedded to the medical stuff and won’t even talk about enhancement because they don’t want to lose their research grants,” says Daniel Faggella , a futurist who founded TechEmergence, a market research firm focusing on cognitive enhancement and the intersection of technology and psychology. But, Faggella says, the technology developed to ameliorate medical conditions will inevitably be put to other uses. “Once we have boots on the ground and the ameliorative stuff becomes more normal, people will then start to say: we can do more with this.”
Doing more inevitably will involve augmenting brain function, which has already begun in a relatively simple way. For instance, scientists have been using electrodes placed on the head to run a mild electrical current through the brain, a procedure known as transcranial direct-current stimulation (tDCS). Research shows that tDCS, which is painless, may increase brain plasticity, making it easier for neurons to fire. This, in turn, improves cognition, making it easier for test subjects to learn and retain things, from new languages to mathematics. Already there is talk of implanting a tDCS pacemaker-like device in the brain so recipients do not need to wear electrodes. A device inside someone’s head could also more accurately target the electrical current to those parts of the brain most responsive to tDCS.
Anders Sandberg, Oxford University’s Future of Humanity Institute
[Smart genes]
According to many futurists, tDCS is akin to an early steam train or maybe even a horse-drawn carriage before the coming of jumbo jets and rockets. If, as some scientists predict, full brain-machine interface comes to pass, people may soon have chips implanted in their brains, giving them direct access to digital information. This would be like having a smartphone in one’s head, with the ability to call up mountains of data instantly and without ever having to look at a computer screen.
The next step might be machines that augment various brain functions. Once scientists complete a detailed map of exactly what different parts of our brain do, they will theoretically be able to augment each function zone by placing tiny computers in these places. For example, machines may allow us to “process” information at exponentially faster speeds or to vividly remember everything or simply to see or hear better. Augments placed in our frontal lobe could, theoretically, make us more creative, give us more (or less) empathy or make us better at mathematics or languages. (For data on whether Americans say they would want to use potential technology that involved a brain-chip implant to improve cognitive abilities, see the accompanying survey, see U.S. Public Wary of Biomedical Technologies to ‘Enhance’ Human Abilities .)
Genetic engineering also offers promising possibilities, although there are possible obstacles as well. Scientists have already identified certain areas in human DNA that seem to control our cognitive functions. In theory, someone’s “smart genes” could be manipulated to work better, an idea that almost certainly has become more feasible with the recent development of CRISPR. “The potential here is really very great,” says Anders Sandberg, a neuroscientist and fellow at Oxford University’s Future of Humanity Institute. “I mean scientists are already working on … small biological robots made up of small particles of DNA that bind to certain things in the brain and change their chemical composition.
“This would allow us to do so many different things,” Sandberg adds. “The sky’s the limit.”
In spite of this optimism, some scientists maintain that it will probably be a long time before we can bioengineer a substantially smarter person. For one thing, it is unlikely there are just a few genes or even a few dozen genes that regulate intelligence. Indeed, intelligence may be dependent on the subtle dance of thousands of genes, which makes bioengineering a genius much harder.
Even if scientists find the right genes and “turn them on,” there is no guarantee that people will actually be smarter. In fact, some scientists speculate that trying to ramp up intelligence – whether by biology or machines – could overload the brain’s carrying capacity. According to Martin Dresler, an assistant professor of cognitive neuroscience at Radboud University in the Netherlands, some researchers believe that “evolution forced brains to develop toward optimal … functioning.” In other words, he says, “if there still was potential to optimize brain functioning by adding certain chemicals, nature would already have done this.” The same reasoning could also apply to machine enhancement, Dresler adds.
Even the optimistic Sandberg says that enhancing the brain could prove more difficult than some might imagine because changing biological systems can often have unforeseen impacts. “Biology is messy,” he says. “When you push in one direction, biology usually pushes back.”
[icon_headline headline=”THE FUTURE OF BLOOD” image=”16104″ align=”aligntop”]
Given the brain’s importance, cognitive enhancement might be the holy grail of transhumanism. But many futurists say enhancement technologies will likely be used to transform the whole body, not just one part of it.
This includes efforts to manufacture synthetic blood, which to this point have been focused on therapeutic goals. But as with CRISPR and gene editing, artificial blood could ultimately be used as part of a broader effort at human enhancement. It could be engineered to clot much faster than natural human blood, for instance, preventing people from bleeding to death. Or it could be designed to continuously monitor a person’s arteries and keep them free of plaque, thus preventing a heart attack.
Synthetic white blood cells also could potentially be programmed. Indeed, like virtually any computer, these cells could receive “software updates” that would allow them to fight a variety of threats, such as a new infection or a specific kind of cancer. 1
Scientists already are developing and testing nanoparticles that could enter the bloodstream and deliver medicine to targeted areas. These microscopic particles are a far cry from synthetic blood, since they would be used once and for very specific tasks – such as delivering small doses of chemotherapy directly to cancer cells. However, nanoparticles could be precursors to microscopic machines that could potentially do a variety of tasks for a much longer period of time, ultimately replacing our blood.
It’s also possible that enhanced blood will be genetically engineered rather than synthetically made. “One of the biggest advantages of this approach is that you would not have to worry about your body rejecting your new blood, because it will still come from you,” says Oxford University’s Sandberg.
Regardless of how it is made, one obvious role for enhanced or “smart” blood would be to increase the amount of oxygen our hemoglobin can carry. “In principle, the way our blood stores oxygen is very limited,” Sandberg says. “So we could dramatically enhance our physical selves if we could increase the carrying capacity of hemoglobin.”
According to Sandberg and others, substantially more oxygen in the blood could have many uses beyond the obvious benefits for athletes. For example, he says, “it might prevent you from having a heart attack, since the heart doesn’t need to work as hard, or it might be that you wouldn’t have to breathe for 45 minutes.” In general, Sandberg says, this super blood “might give you a lot more energy, which would be a kind of cognitive enhancement.”
(For data on whether Americans say they would want to use potential synthetic blood substitutes to improve their own physical abilities, see the accompanying survey, U.S. Public Wary of Biomedical Technologies to ‘Enhance’ Human Abilities .)
[icon_headline headline=”HYPE OR PARADIGM SHIFT?” image=”16105″ align=”aligntop”]
So where is all of this new and powerful technology taking humanity? The answer depends on who you ask.
Having more energy or even more intelligence or stamina is not the end point of the enhancement project, many transhumanists say. Some futurists, such as Kurzweil, talk about the use of machines not only to dramatically increase physical and cognitive abilities but to fundamentally change the trajectory of human life and experience . For instance, Kurzweil predicts that by the 2040s, the first people will upload their brains into the cloud, “living in various virtual worlds and even avoiding aging and evading death.”

Kurzweil – who has done more than anyone to popularize the idea that our conscious selves will soon be able to be “uploaded” – has been called everything from “freaky” to “a highly sophisticated crackpot.” But in addition to being one of the world’s most successful inventors, he has – if book sales and speaking engagements are any indication – built a sizable following for his ideas.
Kurzweil is not the only one who thinks we are on the cusp of an era when human beings will be able to direct their own evolution. “I believe that we’re now seeing the beginning of a paradigm shift in engineering, the sciences and the humanities,” says Natasha Vita-More, chairwoman of the board of directors of Humanity+, an organization that promotes “the ethical use of technology to expand human capacities.”
Still, even some transhumanists who admire Kurzweil’s work do not entirely share his belief that we will soon be living entirely virtual lives. “I don’t share Ray’s view that we will be disembodied,” says Vita-More, who along with her husband, philosopher Max More, helped found the transhumanist movement in the United States. “We will always have a body, even though that body will change.”
George Annas, Boston University
In the future, Vita-More predicts, our bodies will be radically changed by biological and machine-based enhancements, but our fundamental sensorial life – that part of us that touches, hears and sees the world – will remain intact. However, she also envisions something she calls a whole-body prosthetic, which, along with our uploaded consciousness, will act as a backup or copy of us in case we die. “This will be a way to ensure our personal survival if something happens to our bodies,” she says.
Others, like Boston University bioethicist George Annas, believe Kurzweil is wrong about technological development and say talk of exotic enhancement is largely hype. “Based on our past experience, we know that most of these things are unlikely to happen in the next 30 or 40 years,” Annas says.
He points to many confident predictions in the last 30 or 40 years that turned out to be unfounded. “In the 1970s, we thought that by now there would be millions of people with artificial hearts,” he says. Currently, only a small number of patients have artificial hearts and the devices are used as a temporary bridge , to keep patients alive until a human heart can be found for transplant.
More recently, Annas says, “people thought the Human Genome Project would quickly lead to personalized medicine, but it hasn’t.”
Faggella, the futurist who founded TechEmergence, sees a dramatically different future and thinks the real push will be about, in essence, expanding our consciousness, both literally and figuratively. The desire to be stronger and smarter, Faggella says, will quickly give way to a quest for a new kind of happiness and fulfillment. “In the last 200 years, technology has made us like gods … and yet people today are roughly as happy as they were before,” he says. “So, I believe that becoming a super-Einstein isn’t going to make us happier and … that ultimately we’ll use enhancement to fulfill our wants and desires rather than just make ourselves more powerful.”
What exactly does that mean? Faggella can’t say for sure, but he thinks that enhancement of the mind will ultimately allow people to have experiences that are quite simply impossible with our current brains. “We’ll probably start by taking a human version of nirvana and creating it in some sort of virtual reality,” he says, adding “eventually we’ll transition to realms of bliss that we can’t conceive of at this time because we’re incapable of conceiving it. Enhancing our brains will be about making us capable.”
[chapter title=”Ethics and religion” background_image=”16073″]
[icon_headline headline=”A TALE OF TWO HUXLEYS” image=”16100″ align=”aligntop”]

T o some degree, the ideas and concepts behind human enhancement can be traced to biologist and author Julian Huxley. In addition to being one of the most important scientific thinkers of the mid-20th century, Julian also was the brother of Aldous Huxley, author of the famous scientific dystopian novel “Brave New World . ”
The novel is set in a future where, thanks to science, virtually no one knows violence or want. But this brave new world also is a sterile place, where people rarely feel love, where children are “decanted” in laboratories and families no longer exist, and where happiness is chemically induced. Although there is an abundance of material comforts in this fictional world, the things that people traditionally believe best define our humanity and make life worth living – love, close relationships, joy – have largely been eliminated.
In contrast with his brother Aldous, Julian Huxley was a scientific optimist who believed that new technologies would offer people amazing opportunities for self-improvement and growth, including the ability to direct our evolution as a species. No longer, he said, would a person’s physical and psychological attributes be subject to the capricious whims of nature.
[icon_headline headline=”A COST TO SOCIETY?” image=”16101″ align=”aligntop”]
But like Julian’s brother Aldous Huxley, those who oppose radical enhancement say the road to transcending humanity is paved with terrible risks and dangers, and that a society that embraces enhancement might lose much more in the bargain than it gains. “I think that the enhancement imperative, where we’re going to overcome all limitations including death, seems to me to be a kind of utopianism that we’ll have to break a lot of eggs to realize,” says Christian Brugger, a professor of moral theology at St. John Vianney Theological Seminary in Denver.

According to Brugger and other opponents of radical enhancement, those “broken eggs” might include increased social tensions – or worse – as the rich and privileged gain access to expensive new enhancement treatments long before the middle class or poor and then use these advantages to widen an already wide gap between rich and poor. “The risks here of creating greater inequalities seem to be obvious,” says Todd Daly, an associate professor of theology and ethics at Urbana Theological Seminary in Champaign, Ill. “And I’m not convinced that people who get these enhancements will want to make sure everyone else eventually gets them too, because people usually want to leverage the advantages they have.”
For some thinkers, concerns about inequality go much further than merely widening the existing gap between rich and poor. They believe that radical enhancement will threaten the very social compact that underpins liberal democracies in the United States and elsewhere. “The political equality enshrined in the Declaration of Independence rests on the empirical fact of natural human equality,” writes social philosopher Francis Fukuyama in his 2002 book “Our Posthuman Future.” He adds: “We vary greatly as individuals and by culture, but we share a common humanity.”
Brugger of St. John Vianney Theological Seminary agrees. “Right now, there is a common equality because we are all human,” he says. “But all of this changes once we start giving some people significantly new powers.”
Supporters of human enhancement say the goal is not to create a race of superhumans but to use technological tools to improve humanity and the human condition. Indeed, they say, it is an extension of what humans have been doing for millennia: using technology to make life better. “I don’t believe in utopias and I don’t believe in perfection,” says Vita-More, adding that: “For me, enhancement is a very practical way to give us new options to make our lives better. It’s that simple.”
A good example, Vita-More says, is cognitive enhancement. “By giving people increased memory and problem-solving skills, cognitive enhancement will help us be more creative by giving us the ability to put more things together in new ways,” she says. “It will make us better problem solvers.”
James Hughes, Trinity College
The more ability we have as individuals, the better we become.
Those who support human enhancement also deny that these developments will make social inequalities dramatically worse. New technologies are often socially disruptive and can have a negative impact on certain vulnerable populations, they say. But the problem of inequality is essentially, and will remain, a political one.
“The core Luddite mistake is to point to a social problem and to say that if we add new technologies the problem will get worse,” says James Hughes, executive director of the Institute for Ethics and Emerging Technologies, a pro-enhancement think tank. “But the way to cure the problem in this case is to make the world more equal, rather than banning the technology.”
Human enhancement is just as likely, or even more likely, to mitigate social inequalities than to aggravate them, says Oxford University’s Bostrom, a leader in the transhumanist movement. “The enhancement project could allow people who have natural inequalities to be brought up to everyone else’s level,” he says.
Hughes, Bostrom and others also dispute the idea put forth by Fukuyama and Brugger that enhancement could displace the sense of common humanity that has undergirded the democratic social contract for centuries. First, they point out that the history of the modern West has been one of an ever-expanding definition of full citizenship. “The set of individuals accorded full moral status by Western societies has actually increased, to include men without property or noble descent, women and non-white peoples,” Bostrom writes . In addition, supporters of enhancement say, the notion that there will be a distinctive species of enhanced individuals who will try to enslave their unenhanced brothers and sisters might make for good science fiction, but it is not likely to happen. Instead, they say, there will be many different types of people, with different types of enhancements. “It seems much more likely that there would be a continuum of differently modified or enhanced individuals, which would overlap with the continuum of as-yet-unenhanced humans,” Bostrom writes, adding that today there are very different types of people (very tall to very short, very intelligent to intellectually disabled, etc.) who manage to live side by side as moral and legal equals.
Finally, transhumanists and other supporters say, history shows that as people gain more control over their lives, they become more empathetic, not less. “Today we have more health, more intelligence and more lifespan than we did 100 years ago, and we’re more compassionate and more empathetic today then we were then,” Hughes says, pointing to a 2011 book by Harvard University psychology professor Steven Pinker, “The Better Angels of Our Nature: Why Violence Has Declined.” The book makes the case that as human society has grown richer and more sophisticated, it also has become less violent. “The more ability we have as individuals, the better we become,” Hughes adds.
[icon_headline headline=”A COST TO SELF?” image=”16102″ align=”aligntop”]
Christian Brugger, St. John Vianney Theological Seminary
Happiness is found in marriages, in families, in neighborhoods … None of these are promised by enhancement.
Critics of enhancement question whether people really will be happier if enhancement projects are allowed to come to fruition. According to these critics, philosophers have long held that true happiness does not come from enhanced physical prowess or dramatically longer life, but from good character and virtuous living. “Happiness is found in marriages, in families, in neighborhoods … in people who are willing to sacrifice and suffer for others,” Brugger says. “None of these are promised by enhancement.”
“Happiness also is found in limits, says Agar of Victoria University. “There are things that I value and am proud of in my life, like my recent book,” he says. “But how can I value the writing of my book if I’ve been cognitively enhanced, and doing such a thing becomes much easier?”
But supporters contend that life still will be meaningful and challenging in a world where enhancement is widespread. “The things that have to do with human character and virtue and those things that make life meaningful will not change as a result of human enhancement, just like they haven’t changed as our society has changed,” says Ted Peters, a professor of systematic theology at Pacific Lutheran Theological Seminary in Berkeley, California. “As long as we are still human, these things will be important.”
Furthermore, an enhanced life will still contain challenges and limits, just different ones, says Ronald Cole-Turner, a professor of theology and ethics at Pittsburgh Theological Seminary, which is associated with the Presbyterian Church (U.S.A.). “The challenges of life will still be there, they may just be different and harder,” he says. “The goal posts will have moved further down the field, that’s all.”
[icon_headline headline=”TRANSHUMANISM AND FAITH TRADITIONS” image=”16103″ align=”aligntop”]
Because human enhancement is still largely an issue for the future, it has not yet attracted a lot of attention in American religious communities. There is, for instance, no official teaching or statement on human enhancement or transhumanism that has come directly from any of the major churches or religious groups in the United States. However, some theologians, religious ethicists and religious leaders have started to think about the implications of human enhancement in light of their traditions’ teachings, offering a sense of how their churches or religions might respond to radical human enhancement if it became possible.
All of the Abrahamic faiths – Judaism, Christianity and Islam – share the belief that men and women have been created, to some extent, in God’s image. According to many theologians, the idea that human beings in certain ways mirror God make some, but not all, religious denominations within this broad set of connected traditions wary of using new technologies to enhance or change people, rather than heal or restore them.
The Roman Catholic Church, through its large network of educational and other institutions, already has begun formulating an argument against enhancement, based in part on the idea that God’s plan for humanity includes limits and that life’s limits are the very forces that create virtuous, wise and ultimately happy people. “Courage, fidelity, fortitude, generosity, hope, moderation, perseverance, are all cultivated in response to limitations of circumstance and nature,” says John Haldane, a Catholic philosopher who teaches at the University of St. Andrews in Scotland.
Todd Daly, Urbana Theological Seminary
…when we attempt to be something more than human, are we running the risk of trying to become, in some ways, like God, as did Adam and Eve?
Catholics actively support medical and technological advances that can restore someone to health, says Brugger. “But the dividing line for the church is the line between therapy and enhancement.”
Concerns about crossing that line already have been expressed by Catholic-affiliated organizations. In 2013, for instance, the church-affiliated International Science and Life Congress met in Madrid and issued a declaration that warned that “new human species, artificially manipulated” would create “a real danger to human life as we know it.”
[evangelical]
According to Daly and others, evangelicals’ opposition to enhancement would be based in part on the notion that man should not “play God.” According to Daly, “when we attempt to be something more than human, are we running the risk of trying to become, in some ways, like God, as did Adam and Eve?” He adds, “This is an important issue for Christians that, I think, will help drive the debate for us.”
John Haldane, University of St. Andrews
Courage, fidelity, fortitude, generosity, hope, moderation, perseverance, are all cultivated in response to limitations of circumstance and nature.
Opposition also would be likely from the Church of Jesus Christ of Latter-day Saints, which teaches that the body is sacred and thus must not be altered. While small enhancements that do not overtly change the body might be acceptable to Mormon leaders, more significant enhancements would probably be “seen as a problem by the church,” says Steven Peck, a bioethicist at Brigham Young University in Provo, Utah.
The Hindu tradition probably would approach human enhancement as a potentially dangerous development as well, although for different reasons than Christian churches, says Deepak Sarma, a professor of South Asian religions and philosophy at Case Western Reserve University in Cleveland. Enhancement is troubling, he says, because it could be used to alleviate suffering, which is necessary to work off bad karma (debt from bad deeds and intents committed during a person’s past lives). Viewed in this light, Sarma says, Hindus could see enhancement as keeping someone from cleansing themselves of these misdeeds from their past lives.
In Islam, according to Sherine Hamdy, an associate professor of anthropology at Brown University, human enhancement would be viewed with concern by some scholars and leaders and embraced by others. Supporters might see new enhancements as a way to help the Muslim world catch up with the West or “at least not get left further behind,” she says. Others would oppose enhancements out of a desire “not to change what God has created.”
According to Lutheran theologian Peters, many mainline churches will view enhancement positively because they will see aspects of it as attempts to improve human well-being and alleviate suffering. “I think they will see much of this for what it is: an effort to take advantage of these new technologies to help improve human life,” he says.
Hava Tirosh-Samuelson, Arizona State University
So long as the improvement alleviates or prevents suffering, it is inherently good …
Similarly, Buddhists would largely accept and even embrace human enhancement because it could help them become better Buddhists, says Hughes, who is an advocate for transhumanism as well as a Buddhist and a former Buddhist monk. Enhancement that extends life and makes people more intelligent “would be seen as good because you’d have more time to work on enlightenment and … you could be more effective in helping others,” he says.
[in Jewish law]
In spite of intense disagreements about the utility and morality of trying to “improve” humanity, many thinkers on both sides of the debate share the belief that if just some of the dreams of today’s transhumanists are realized, human society will change and change significantly. These changes, if they occur, will upend some social norms and possibly religious norms as well. And they will force churches and many other institutions (both religious and secular) to adjust to a new reality. For the first time in human history, the biggest material changes in our society may not be occurring outside of ourselves, in the fields, factories and universities that have shaped human civilization, but inside our bodies – in our brains and muscles and arteries, and even in our DNA.
- Kurzweil, Ray and Terry Grossman. 2004. “Fantastic Voyage: Live Long Enough to Live Forever,” Pp. 226-227. ↩
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
Sign up for The Briefing
Weekly updates on the world of news & information
Most Popular
901 E St. NW, Suite 300 Washington, DC 20004 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan, nonadvocacy fact tank that informs the public about the issues, attitudes and trends shaping the world. It does not take policy positions. The Center conducts public opinion polling, demographic research, computational social science research and other data-driven research. Pew Research Center is a subsidiary of The Pew Charitable Trusts , its primary funder.
© 2024 Pew Research Center

Speaking Truth to Israel Requires More Than Academic Freedom

Payangko, or Echidna ( Zaglossus attenboroughi )

Inside Amazon’s Union-Busting Tactics

Fighting for Reproductive Rights in Retirement

Can Ancient DNA Support Indigenous Histories?

Can Embracing Copies Help With Museum Restitution Cases?

Can Art Save the “Post-Apocalyptic” Salton Sea?

How Allocating Work Aided Our Evolutionary Success

Bringing Back the World’s Most Endangered Cat

The Shortcomings of Height in Politics

Griko’s Poetic Whisper

When a Message App Became Evidence of Terrorism

The Rise of Aunties in Pakistani Politics

For Families of Missing Loved Ones, Forensic Investigations Don’t Always Bring Closure

Albania’s Waste Collectors and the Fight for Dignity

Grappling With Guilt Inside a System of Structural Violence

Inside Russia’s Campaign to Steal and Indoctrinate Ukrainian Children

Coastal Eden

On the Tracks to Translating Indigenous Knowledge

A Call for Anthropological Poems of Resistance, Refusal, and Wayfinding

Buried in the Shadows, Ireland’s Unconsecrated Dead

Nameless Woman

A Palestinian Family’s History—Told Through Olive Trees

Can “Made in China” Become a Beacon of Sustainability?

The International Order Is Failing to Protect Palestinian Cultural Heritage

Spotlighting Black Women’s Mental Health Struggles

Being a “Good Man” in a Time of Climate Catastrophe

Cultivating Modern Farms Using Ancient Lessons

Imphal as a Pond

A Freediver Finds Belonging Without Breath

The Trauma Mantras

The Ethical Battle Over Ancient DNA

In February, scientists published a critically important contribution to our understanding of the ancient Pueblo peoples of the American Southwest. A 14-member interdisciplinary team, including anthropologists, archaeologists, and geneticists, succeeded in extracting and sequencing DNA from the remains of numerous individuals who were apparently elite people at Chaco Canyon, the wondrous archaeological complex in northwest New Mexico. In its heyday, about A.D. 800 to A.D. 1150, Chaco was at the center of one of the richest and most sophisticated civilizations to grace North America before European colonists arrived in the 16th century. Its cultural influence spread throughout what is today known as the Four Corners region of New Mexico, Arizona, Utah, and Colorado, and possibly as far south as modern-day Mexico.
The findings, which provide important insights into the social structure of the ancient Puebloans, have sparked considerable excitement among archaeologists and others who study the ancient past. One might think their enthusiasm would be shared by present-day Pueblo peoples, who claim the Chacoans as their ancestors—a contention strongly backed by archaeological evidence.
But instead, news of the research, which was published in the journal Nature Communications , has made some Native American tribal officials very angry. In contrast to other recent cases in which scientists have consulted tribal peoples and obtained their blessings before extracting DNA from ancestral Native American remains, the researchers are only now discussing their results with tribal groups. To a number of critics, including some researchers, the controversy represents a setback to recent progress in fostering collaborations between scientists and tribal peoples.
“It is clearly ethically problematic to carry out destructive analyses on Indigenous human remains without talking to any Native peoples about it,” says archaeologist Ruth Van Dyke, a Chaco researcher at Binghamton University in upstate New York. “In recent decades, archaeologists have been working hard to build trusting, collaborative relationships with our Indigenous colleagues. Any research that fails to respect Native rights and sensibilities can only undermine this progress.”
The episode is all the more troubling because it involves a renowned institution, the American Museum of Natural History (AMNH), which has held these ancient Puebloan remains in its collection since they were first excavated in the late 1890s. Yet the museum has been tight-lipped, both about the basis on which it granted the team permission to carry out this recent research as well as about an earlier review, formalized in 2000, of the legal status of these human remains. It has released only a brief statement and declined to make public the detailed documentation that supposedly backed up these decisions. Nevertheless, this author has obtained the 2000 document, a detailed inventory and review of the remains that was mandated under federal law. The AMNH’s review, which was conducted during the late 1990s, concluded that the bones were “culturally unidentifiable”—that is, they could not be linked directly to any living tribal group.
The museum seems to have fulfilled at least the letter of the law. The law that mandated the review, the 1990 Native American Graves Protection and Repatriation Act (NAGPRA), requires all public and private museums that receive federal funds (except the Smithsonian Institution) to inventory Native American artifacts and human remains in their possession and to consult with tribal groups that might want to claim them. In its statement to the author, the AMNH asserted that during the latter half of the 1990s it had attempted to contact southwestern tribes about the human remains it held but that none had made a claim of affiliation with them.

Museum officials declined to elaborate on exactly who they had contacted and how hard they had tried, however. In practice, the law gives institutions a great deal of latitude in how they interpret its requirements. Thus tribal groups have sometimes had to go to court to prove that they are culturally affiliated with particular human remains or cultural items in museum collections. Although current federal regulations interpreting NAGPRA dictate that the tribes can win such cases if the “preponderance of the evidence” leans in their favor, even that somewhat generous standard can sometimes be hard for tribal groups to meet.
But if the AMNH fulfilled the letter of the law, the question remains whether it fulfilled the law’s spirit of respect for tribal cultural traditions. The museum submitted its review to NAGPRA authorities in June of 2000. A great deal has happened since, including the repatriation and reburial of the famed Kennewick Man—known as the Ancient One to the Native American tribes who claimed him—after nearly 20 years of battles between tribal groups and some scientists. Some anthropologists had argued, based on analysis of his skull shape, that Kennewick Man was most closely related to people from Polynesia or the Ainu ethnic group of Japan, and not genetically related to contemporary Native Americans. In that case, ironically, the DNA results provided key evidence that the 8,500-year-old Ancient One, who was found in 1996 by the Columbia River in the state of Washington, was indeed genetically close to present-day Native Americans, including one of the local tribes that was claiming him.
But the AMNH apparently made little or no attempt to bring its 2000 review up to date. Prior to approving the new DNA research, the museum, according to its own statement, did not revisit the review’s nearly 20-year-old conclusions, nor did it consult with either contemporary Pueblo groups or representatives of the Navajo Nation, which today surrounds the federally protected Chaco Culture National Historical Park. (The Navajo also claim the Chaco people as their ancestors, although that contention is seen as controversial by some.) The AMNH’s explanation for not consulting with the tribes before granting the team permission to do its research is “unacceptable” given NAGPRA’s long history, says Kurt Dongoske, the tribal historic preservation officer for the Pueblo of Zuni in northwest New Mexico. “It has been 27 years since the passage of NAGPRA, and a lot of tension, disagreements, learning, and cultural and historical sensitivity training has been experienced by tribes, museums, and federal agencies.”
It’s understandable that researchers would want to find out as much as they can about the far-flung “Chaco world” and its rich architecture and culture. Here in Chaco Canyon, under New Mexico’s bluer-than-blue sky, visitors can walk among the haunting ruins of this great civilization. More than a dozen massive “great houses” line the canyon, each constructed with skillfully cut wooden beams and intricately laid stone bricks that would be the pride of modern carpenters and masons.
During an 1896 expedition, archaeologists broke into a small burial crypt in the most spectacular of these great houses, called Pueblo Bonito, which had more than 600 rooms and was, in one part, four stories high. They found 14 individuals crammed into the crypt. The two earliest burials were adorned with thousands of turquoise beads, and other human remains were associated with artifacts that included pottery and delicately carved flutes and ceremonial staffs. Archaeologists have long suspected that these individuals belonged to an elite group because more turquoise was found in this one crypt than in most other southwestern sites put together.
For the new study, the research team, led by archaeologists Douglas Kennett of Pennsylvania State University and Stephen Plog of the University of Virginia, directly radiocarbon dated the bones of 11 of the burials and extracted DNA from nine of them. The DNA sequences revealed a long-hidden surprise : The Pueblo Bonito elite all shared the same maternal ancestor, whose DNA had been passed down from generation to generation over more than 300 years. This so-called matrilineal inheritance, the research team pointed out, is similar to the traditional social structure of the Zuni, Hopi, Acoma, and other Pueblo groups of the Southwest. These DNA results could bolster existing archaeological evidence that present-day Puebloans are the direct descendants of the ancient Chacoans, especially if some tribal people were now willing to donate DNA samples for comparison. Indeed, such further studies might provide genetic evidence for the cultural affiliation between Chaco peoples and modern Puebloans that the AMNH’s review in 2000 failed to find.

Just such genetic evidence clinched the case of Kennewick Man, whose 8,500-year-old genome was sequenced by ancient DNA expert Eske Willerslev, from Denmark, and his colleagues. Before extracting and analyzing the ancient DNA, however, Willerslev began a lengthy consultation with the five Washington state tribes who had claimed the Ancient One, in vain, for nearly two decades. Willerslev urged them to donate their own DNA samples for the study. While only one tribe, the Colville, agreed to do so, providing two dozen samples, Willerslev’s group found not only that the Ancient One was a true Native American but that the Colville were closely related to him. Although some experts questioned just how close that relationship really was, and whether it was intimate enough for the Colville to claim he was their direct ancestor, the U.S. Army Corps of Engineers, which had formal custody of Kennewick Man, was apparently convinced by the DNA evidence . Last December, President Barack Obama signed legislation passed by Congress authorizing the return of the Ancient One to the tribes, and on February 18, he was buried in a secret location not far from where he was found.
“The time when scientists should go and study ancient human remains from the Americas without some kind of tribal engagement has passed,” Willerslev says. “For many tribal groups, human remains from the Americas are considered ancestors whether or not there is evidence of cultural or genetic links.” Ignoring tribal consultation, he adds, “can no longer be explained by lack of awareness. It’s a decision, and must be considered a statement.”
Most importantly, a growing number of researchers are realizing that the strong feelings Native Americans have about the remains of their ancestors do not reflect an anti-science perspective. Native Americans are keenly interested in their history and prehistory, and, under the right circumstances, they have been increasingly willing to cooperate with scientific studies—if, that is, scientists respect their traditions and do not fall back on earlier colonialist attitudes that allowed archaeologists to strip whatever they wanted from ancient sites.
In a joint statement released shortly before their paper was published, the scientists on the research team—all of whom are based in institutions on the U.S. East Coast—said that they had relied on the AMNH’s determination that the Chaco human remains could not be traced to specific tribes. In a later statement to this author, Kennett said that “the AMNH specifically requested that they be the ones to handle” the cultural affiliation issues, adding that his team had “every reason to think that the AMNH were careful about this and they have great expertise in this area and we deferred to them here.”
In the museum’s own statement, the AMNH said that the team’s research proposal had been reviewed by its Anthropology Loan Committee and approved because “the research had considerable scientific merit with little impact on the artifacts and human remains,” adding that during its 1990s review no tribes “came forward to claim affiliation.”
However, the AMNH’s 1990s review contains no evidence that the museum attempted to consult with tribal groups about potential affiliations with the Chaco human remains it held, despite NAGPRA’s requirement that it do so. Rather, the 14-page document, which lists details about all of the Chaco remains in its possession, argues in general terms that “while most or all pueblos may have some association with Ancestral Puebloan peoples of [New Mexico’s] San Juan Basin, including similarities in ceramics, architecture, and subsistence practices, the nature of that connection is not sufficiently clear to warrant making a determination of ‘cultural affiliation’ within the meaning of NAGPRA to all the Puebloan groups in New Mexico and Arizona.”
The AMNH has declined to discuss these conclusions beyond its original brief statement. However, Leigh Kuwanwisiwma, who has been the director of cultural preservation for the Hopi Tribe in Arizona since 1989, says that the museum never contacted him or the Hopi Tribe during its 1990s review. That is all the more surprising because at that time Chaco national park was engaged in an intensive, controversial, and very public consultation with all of the Pueblo tribes and the Navajo over the cultural affiliation of more than 280 human remains in its own collection. Eventually, the National Park Service determined that the remains were affiliated with more than 20 Pueblo tribes and the Navajo, a decision upheld by the U.S. Department of the Interior. In 2006, the Park Service handed the remains over to tribal groups, who reburied them in a secret location in Chaco Canyon. (Other museums, including the Museum of Natural History at the University of Colorado, Boulder, have come to similar conclusions about Chaco remains in their possession.)
In the current case of the Pueblo Bonito burials, Kuwanwisiwma says, the news of the ancient DNA results came as a complete surprise. “Absolutely no one told me” that ancient DNA would be extracted from the remains, he says. “The tribe never gave any kind of permission or support.” Kuwanwisiwma says the matter is now being discussed among some of the Pueblo groups, particularly the Hopi, Zuni, Acoma, and Santa Clara pueblos, and that the All Pueblo Council of Governors has also taken up the matter. “It has clearly been the position of tribes in the U.S., and particularly in the Southwest, that we have never supported destructive DNA analysis.”

As for whether Native Americans might eventually cooperate with the research team, for example by providing DNA samples for comparison, as the Colville Tribe did in the case of Kennewick Man, Kuwanwisiwma says it is too soon to know. That decision, he adds, would depend on whether the Hopi and other Pueblo groups would ultimately benefit from such a step.
And for Dongoske from the Pueblo of Zuni, the central issue is whether the tools of science necessarily trump the traditional knowledge of Indigenous peoples passed down over the ages. “Native Americans do not need scientists to tell them their heritage,” he says. “Their heritage has been passed down to them in oral history, through the recitation of prayers, and in ceremonial traditions. To claim that the Western knowledge system, science, is the only legitimate way of knowing the past is insulting to all of us and it perpetuates the insidious effects of colonialism on Native Americans.”
Can science and Indigenous cultural traditions be reconciled? Perhaps they cannot—or at least not in every case and not entirely. But the story of the Ancient One and other recent cases of collaboration between ancient DNA researchers and Native Americans show that the interests of both groups can sometimes overlap. Indeed, there are signs that at least some of the Chaco team members are now realizing that. “I personally wish that as authors we had communicated earlier on in the study with potentially interested Native American pueblos and tribes to obtain their perspectives in addition to our interactions with the AMNH,” says George Perry, the Pennsylvania State University paleogeneticist who conducted the principal ancient DNA analyses for the team.
Such engagement with tribal groups may well be necessary if the team is to continue its research, which could involve possible ancient DNA analysis of other human remains from Pueblo Bonito, most of which are held by the Washington, D.C.–based Smithsonian Institution. But real engagement and consultation means that museums must be willing to take the risk that their long-held collections of Native American remains—which some living peoples consider to be those of their ancestors—may have to be given back and returned to the Earth from which they came.
Correction: April 3, 2017 An earlier version of this article stated that the Chaco civilization ’ s cultural influence spread throughout the Four Corners region of New Mexico, Arizona, Utah, and Colorado, and possibly as far south as Mexico. This has been updated to make it clear that its influence spread throughout what is today known as the Four Corners region and modern-day Mexico.

Michael Balter is a freelance writer and reporter who covers anthropology, archaeology, mental health, and sexual harassment in the sciences. His work has appeared in Science , Scientific American , Audubon , The Verge , and many other publications. He is also the author of The Goddess and the Bull: Çatalhöyük, an Archaeological Journey to the Dawn of Civilization . Follow him on Twitter at @mbalter .

Stay connected
Facebook , Instagram , LinkedIn , Threads , Twitter , Mastodon , Flipboard
Y ou may republish this article, either online and/or in print, under the Creative Commons CC BY-ND 4.0 license. We ask that you follow these simple guidelines to comply with the requirements of the license.
I n short, you may not make edits beyond minor stylistic changes, and you must credit the author and note that the article was originally published on SAPIENS.
A ccompanying photos are not included in any republishing agreement; requests to republish photos must be made directly to the copyright holder.
We’re glad you enjoyed the article! Want to republish it?
This article is currently copyrighted to SAPIENS and the author. But, we love to spread anthropology around the internet and beyond. Please send your republication request via email to editor•sapiens.org.
Accompanying photos are not included in any republishing agreement; requests to republish photos must be made directly to the copyright holder.

Request an Essay
Follow class ace for product announcements and ai tips & tricks:.


IMAGES
VIDEO
COMMENTS
A version of this article appears in print on , Section D, Page 4 of the New York edition with the headline: Essay Extends Debate Over DNA Discovery. Order Reprints | Today's Paper | Subscribe.
DNA at 70: Untangling Rosalind Franklin's Role. Historians have long debated the role that Dr. Franklin played in identifying the double helix. A new opinion essay argues that she was an "equal contributor.". On April 25, 1953, James Watson and Francis Crick published a landmark paper in Nature, proposing the double helix as the long ...
Yet debate over her role in the discovery of DNA's structure and her failure to be recognized for it began simmering after the publication of Watson's bestselling book The Double Helix: A ...
April 25, 2024 DNA Day. 1st Place: Megan Xie, Grade 12. Teacher: Mrs. Margot Bram. School: Lower Moreland High School. Location: Huntingdon Vy, Pennsylvania. The early years of genetics centered around the central dogma of biology, the theory that genes in our DNA encode RNA to make proteins. Proteins execute a broad range of functions that ...
ASHG is proud to support National DNA Day through the Annual DNA Day Essay Contest. DNA Day commemorates the completion of the Human Genome Project in April 2003 and the discovery of the double helix of DNA in 1953. This contest is open to students in grades 9-12 worldwide and asks students to examine, question, and reflect on important ...
The structure of DNA. In the early 1950s, the identity of genetic material was still a matter of debate. The discovery of the helical structure of double-stranded DNA settled the matter — and ...
The average science novice might point to the Human Genome Project that had roots in the 1980s as the origin of modern DNA science. But it goes back further than that, to the discovery of the double helical structure in the 1950s and the development of the sequencing process in the 1970s that unlocked the genetic information contained in DNA.
Z-DNA is a transient form of DNA, only occasionally existing in response to certain types of biological activity (Figure 5). Z-DNA was first discovered in 1979, but its existence was largely ...
How DNA's discovery changed the world - and my life. Video, 00:05:43 How DNA's discovery changed the world - and my life
An undamaged strand from the double-stranded homolog pairs with the resected region and serves as a template for DNA synthesis. The crossed over strands form a Holliday junction which can be resolved in different ways. Szostak et al. Nonhomologous end joining: DNA with both strands broken by external agents or during the immune response
Essay Extends Debate Over DNA Discovery Two authors argue that Rosalind Franklin was an 'equal contributor.' By EMILY ANTHES On April 25, 1953, James Watson and Francis Crick published a landmark paper in Nature, proposing the double helix as the long elusive structure of DNA, a discovery that a decade later earned the men the Nobel Prize ...
The structure of DNA double helix and how it was discovered. Chargaff, Watson and Crick, and Wilkins and Franklin. Skip to main content. ... Lesson 2: Discovery of DNA. DNA as the "transforming principle" Hershey and Chase: DNA is the genetic material. Classic experiments: DNA as the genetic material ...
The internal debates about ethics within the family history community suggest that those in the field will need to work hard to establish working practice over the next decades, but, further, that family history as a community needs to take care to develop ways of protecting this information. 135 This debate has become hardened in the last few ...
DNA polymerase, an enzyme discovered in 1955, has the remarkable capacity to catalyze the template-directed synthesis of DNA (1, 2). The discovery of DNA polymerase has contributed in major ways to our present day understanding of how DNA is replicated and repaired and how it is transcribed. It also permitted the development of PCR and DNA sequencing, upon which much of modern biotechnology is ...
The potential benefits of DNA profiling are clear. When two DNA profiles do not match, it suggests that the DNA samples were derived from different individuals; it provides exclusionary evidence [1].Post-conviction DNA comparison has helped to exonerate many from wrongful convictions [2].Forensic DNA analysis, based on the analysis of a single or simple mixed DNA sample, has become the gold ...
The protein backers had a very simple philosophy: DNA is composed of just 4 nucleotides but proteins are composed of 20 amino acids (see essay IX). They reasoned that like an alphabet composed of 20 letters could spell more words than one with 4 letters, so proteins could account for greater genetic variability (diversity).
Dna Discovery Essay. 722 Words 3 Pages. The discovery that genetic information was passed on through DNA as well as the physical structure of DNA being a double helix, containing two complementary strands of DNA were some of the most significant biological discoveries in recent history. ... Medical devices have transformed over the years, much ...
Essay: The Scientific and Ethical Dimensions of Radical Life Extension (2013) According to Brugger and other opponents of radical enhancement, those "broken eggs" might include increased social tensions - or worse - as the rich and privileged gain access to expensive new enhancement treatments long before the middle class or poor and ...
2 scholars extend scrutiny over role of female scientist in identifying double helix 2023-05-24 - BY EMILY ANTHES On April 25, 1953, James Watson and Francis Crick published a landmark paper in Nature, proposing the double helix as the long elusive structure of DNA, a discovery that a decade later earned the men the Nobel Prize in Physiology ...
DNA looks like a twisted ladder wherein rungs are secured by two out of four molecules that are interlocking. These molecules are nucleic acid bases. The four molecules include thymine, adenine, cytosine, and guanine (University of Georgia, 2007). Certain scientists have been notable for conducting experiments leading to the discovery of DNA.
In February, scientists published a critically important contribution to our understanding of the ancient Pueblo peoples of the American Southwest. A 14-member interdisciplinary team, including anthropologists, archaeologists, and geneticists, succeeded in extracting and sequencing DNA from the remains of numerous individuals who were apparently elite people at Chaco Canyon, the wondrous ...
The discovery of DNA, the fundamental building block of life, has had a profound impact on both scie... Open Menu. Close Menu. Answers. Login. Free Trial. AI Tools. Answers. Boards Lessons Apps Membership. Login. Free Trial. AI Essay Writer. Write an essay about The Impact of the DIscovery of DNA on Science and Society. Asked on 12/19/2023, 2 ...
the concept of junk DNA — long espoused by evolutionists — has overall been refuted by mountains of data. and. A major Nature paper by the ENCODE consortium reported evidence of "biochemical functions for 80%" of the human genome. Lead ENCODE scientists predicted that with further research, "80 percent will go to 100" since ...