An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


The impact of needle and syringe exchange programs on HIV-related risk behaviors in low- and middle-income countries: A systematic review and meta-analysis examining individual- versus community-level effects
Ping teresa yeh, caitlin e kennedy, kevin a armstrong, virginia a fonner, kevin r o’reilly, michael d sweat.
- Author information
- Article notes
- Copyright and License information
Co-first authors:
Authors’ contributions: MDS and KRO conceptualized the study. MDS, KRO, PTY, CEK, and VAF were responsible for the methodology of the study. PTY and XY were responsible for data curation, though all authors were involved in literature search and screening, data collection, and data analysis to varying degrees. KAA and MDS conducted formal meta-analysis, while PTY, XY, S, and CEK performed qualitative synthesis. The original draft was written by PTY and XY, with support from S and CEK. All authors were involved in reviewing and editing the manuscript and gave their approval for submitting the final manuscript.
Corresponding author: Ping Teresa Yeh, 615 N Wolfe St, Baltimore, MD 21205 USA, [email protected]
Issue date 2023 Oct.
We conducted a systematic review and meta-analysis of the impact of needle and syringe exchange programs (NSP) on both individual- and community-level needle-sharing behaviors and other HIV-related outcomes in low- and middle-income countries (LMIC). A search of five databases for peer-reviewed trial or quasi-experimental studies reported through July 2021 identified 42 interventions delivered in 35 studies, with a total of 56,751 participants meeting inclusion criteria. Random-effects meta-analysis showed a significant protective association between NSP exposure and needle-sharing behaviors at the individual-level (odds ratio [OR]=0.25, 95% confidence interval [CI]= 0.16–0.39, 8 trials, n=3,947) and community-level (OR=0.39, CI=0.22–0.69, 12 trials, n=6,850), although with significant heterogeneity. When stratified by needle-sharing directionality, NSP exposure remained associated with reduced receptive sharing, but not distributive sharing. NSP exposure was also associated with reduced HIV incidence and increased HIV testing but there were no consistent associations with prevalence of bloodborne infections. Current evidence suggests positive impacts of NSPs in LMICs.
Keywords: HIV prevention, meta-analysis, needle/syringe exchange program (NSP), people who inject drugs (PWID), systematic review
Realizamos una revisión sistemática y un metanálisis del impacto de los programas de intercambio de agujas y jeringas (NSP, por sus siglas en inglés) de los comportamientos de uso compartido de agujas tanto a nivel individual como comunitario y otros resultados relacionados con el VIH en países de ingresos bajos y medianos (LMIC, por sus siglas en inglés). Realizamos búsquedas sistemáticas en cinco bases de datos hasta julio de 2021 en busca de ensayos revisados por pares o estudios cuasiexperimentales. En general, 42 intervenciones informadas en 35 estudios entre 56 751 participantes cumplieron los criterios de inclusión. El metanálisis de efectos aleatorios de ocho estudios a nivel individual y 12 a nivel comunitario con 11 075 participantes en total mostró una asociación protectora significativa entre la exposición a NSP y los comportamientos de compartir agujas (individual: OR = 0,25, IC del 95 %: 0,16–0,39; comunidad: OR=0,39, IC95%:0,22–0,69), aunque con una heterogeneidad importante. Cuando se estratificó por la direccionalidad del intercambio de agujas, la exposición a NSP permaneció asociada con un intercambio receptivo reducido, pero no con un intercambio distributivo. La exposición a NSP también se asoció con una incidencia reducida del VIH y un aumento de las pruebas del VIH, pero no hubo asociaciones consistentes para la prevalencia de infecciones transmitidas por la sangre. La evidencia actual sugiere impactos positivos de los NSP en los LMIC.
Introduction
Globally, there are approximately 15.6 million people who inject drugs according to a systematic review published in 2017. 1 People who inject drugs are at increased risk for contracting HIV, hepatitis B, hepatitis C, and other bloodborne diseases. 2 In many low- and middle-income countries (LMIC), globalization has brought both positive changes, such as improved transportation and international trade, and challenges, such as dissemination of illicit drugs and facilitation of HIV transmission. 3 Risk factors associated with HIV infection among people who inject drugs include poor health literacy, low health awareness, limited access to clean needles or other sterile drug injecting equipment, and the practice of sharing needles/syringes in groups. 4 , 5 In addition, people who inject drugs are often stigmatized and face discrimination, especially in LMICs where the practice of injecting drugs is typically illegal, creating further barriers to care-seeking. 6
Over the past 30 years, many countries have sought to reduce the risk of disease transmission from sharing contaminated needles through the adoption of harm reduction programs. These programs may include needle and syringe exchange programs (NSP), which strive to reduce high-risk behaviors like needle-sharing and needle reuse, 5 , 7 among other interventions including overdose prevention, safe injection sites, and opioid substitution therapy. NSPs reduce drug injection-related harm by providing sterile drug injection equipment and a location for safe syringe disposal, and they also often offer other risk reduction interventions, such as condom promotion/provision and other HIV-related services. 8
Multiple systematic reviews have shown that NSPs are effective in reducing HIV transmission among people who inject drug in high-income settings. 5 , 9 , 10 Despite stigma and criminalization of drug use, which impede NSP uptake, 11 there is now substantial evidence supporting the effectiveness of NSPs in LMICs as well. 12 However, the most recent reviews of the impact of NSPs in LMICs included studies published through 2011; there is thus a need to document the current state of the published evidence for NSPs. Further, previous reviews have not clearly distinguished between the impact of NSPs on individual-level versus community-level outcomes. At the individual-level, studies may compare individuals who use NSP services to other individuals who do not; this demonstrates the effect of NSPs on those who use them. At the community level, studies may compare communities where NSP services are available to other communities where such services are not available; this demonstrates the broader effect of NSPs, not only on those who use them, but also on the wider community. Separately examining the evidence for an impact at both levels allows us to better understand the full impact of NSPs.
We sought to evaluate the impact of NSPs on both individual- and community-level needle-sharing behaviors and other HIV-related outcomes in LMICs. This research was conducted as part of a larger series of systematic reviews conducted by the Evidence Project evaluating the effectiveness of behavioral interventions for HIV in LMICs. We conducted this review in accordance with PRISMA guidelines. 13
For the purposes of this review, we defined NSPs as programs that (1) provide sterile injecting equipment including needles and syringes to people who inject drugs, whether the needles and syringes are sold, exchanged, or given freely, and (2) help dispose of used needles and syringes safely.
Eligibility Criteria
Studies were included in the review if they met the following criteria:
data were presented from a LMIC, defined by combining the World Bank classifications 14 of low-income, lower-middle-income, or upper-middle-income economies at the time of the study;
needles and/or syringes were distributed, exchanged, or sold to people who inject drugs as part of the intervention;
the study population were people who inject drugs, according to any definition used by study authors (e.g. ever, past year, or other time frames);
an evaluation design was employed that compared post-intervention outcomes using either a pre/post or multi-arm study design (this could include randomized trials, non-randomized trials, observational studies, before-after studies, cross-sectional, or serial cross-sectional analyses);
specific outcomes of interest including use of new/sterile needles/syringes, return of used needles/syringes, needle borrowing/sharing, and other behavioral, psychological, social, care or biological outcome(s) related to HIV prevention were presented; and
the article was published in a peer-reviewed journal.
No language restrictions were used. When an article in a language other than English was found, it was translated into English for full-text review.
Search Strategy
We searched five electronic databases (PubMed, PsycINFO, Sociological Abstracts, the Cumulative Index to Nursing & Allied Health Literature (CINAHL), and EMBASE) for articles published from January 1, 1990 through July 25, 2021. We used the following search terms, adapted for each database ( Appendix A ): (“needle exchange” OR “needle distribution” OR “needle sales” OR “syringe sales” OR “syringe distribution” OR “syringe exchange” OR “syringe-exchange” OR “needle-exchange” OR “needle syringe programs” OR “needle syringe program” OR “needle syringe programme” OR “needle exchange programmes”) AND (HIV or AIDS).
To identify articles not obtained from electronic database searching, study staff hand-searched the table of contents of the following journals: AIDS , AIDS and Behavior , AIDS Care, AIDS Education and Prevention, and the International Journal of Drug Policy . Finally, we examined the reference lists of articles selected to further identify potential articles for inclusion. This process was iterated until no new articles were found.
Initial screening of studies was done by a member of the study staff, who excluded clearly non-relevant articles based on the titles and abstracts. Two study staff members conducted secondary screening, independently reviewing the abstracts of the remaining citations for inclusion. Differences in screening decisions were discussed to establish consensus. The final inclusion/exclusion of studies identified articles to be included in the qualitative synthesis and quantitative meta-analysis based on a thorough reading of the full-text article.
Data Extraction and Management
Each article meeting the inclusion criteria underwent data abstraction by two study staff members working independently. Data were entered into a standardized, detailed coding form including the following information:
study year, study location, and study design;
gender and age distribution, sample population, sample size, sampling strategy, loss to follow up, and comparison groups;
description of the NSP intervention (name of the program, recruitment strategy, facilitator/role of implementer, ways of exchange/distribution site, and intervention activities);
description of the outcome reported (needle/syringe needle sharing, other HIV- or sexually transmitted infection (STI)-related outcomes);
outcome measures, statistical tests used, and steps taken to control for covariates;
intervention effects and findings.
Completed coding forms from the two study staff members were compared for discrepancies, and differences were resolved through discussion and consensus with a third reviewer from the senior study staff.
Quality assessment
Rigor of included articles was assessed using an eight-item risk of bias tool developed for the larger series of systematic reviews by the Evidence Project, 15 conducted independently by two reviewers. Differences were resolved by consensus. The items were: (1) prospective cohort; (2) control or comparison group; (3) pre/post intervention data; (4) random assignment of participants to the intervention; (5) random selection of subjects for assessment; (6) follow-up rate of 80% or more; (7) comparison groups are equivalent on socio-demographic measures; and (8) comparison groups are equivalent at baseline on outcome measures.
Data Analysis
We focused on categorical outcomes of needle/syringe sharing behaviors and NSP exposure. We extracted count and proportion data that evaluated the association between NSP exposure and needle/syringe sharing behaviors based on between-group or pre-post comparisons. In meta-analysis, we only included studies that directly examined the association between needle/syringe sharing behaviors and NSP exposure. Adjusted effect sizes and 95% confidence intervals (CIs) for needle/syringe sharing behaviors associated with exposure to NSP were chosen over crude ones when available. Otherwise, the number and proportion of participants reporting the outcome of interest in both intervention and control/comparison groups or comparing before and after NSP exposure were extracted from each study. We then calculated the study-specific effect size expressed as an unadjusted odds ratio (OR) based on the available count and proportion data. For outcomes measured at multiple time points following NSP exposure, we used the last measurement period unless a study included a longer follow-up than most but also included a time point more similar to the remaining studies. When a study was conducted in two cities or countries and presented data separately, we considered them as separate interventions, so we calculated two location-specific effect sizes.
Meta-analysis was performed using Comprehensive Meta-Analysis, version 3 (CMA; Biostat, Englewood, NJ), using random-effects models to calculate pooled OR estimates and 95% confidence intervals. We assessed statistical heterogeneity using both Q and I 2 statistics. In the presence of substantial heterogeneity, we conducted sub-group analyses based on whether there was individual- or community-level outcome measurement and the type of needle/syringe-sharing behavior. First, we classified studies according to individual- or community-level outcome measurement. Separate analyses were conducted based on type of needle/syringe-sharing behavior: receptive sharing (i.e., receiving, borrowing, or reusing other persons’ used needles/syringes) and distributive sharing (i.e., distributing, lending, or passing on any used needles/syringes to other persons). Indirect sharing behaviors (e.g., backloading or frontloading needles/syringes 22 ) were excluded because few studies reported these outcomes. A third analysis combined both outcomes if a study reported both receptive and distributive sharing. In addition, if a study presented needle/syringe-sharing outcomes without specifying sharing directionality, we reported the non-directional estimates of effect sizes as a fourth analysis. Finally, we combined all study-specific estimates (including receptive, distributive, and reported non-directional sharing results) to assess overall NSP impact. We also created funnel plots of standard error by log odds ratio to assess potential reporting bias.
We excluded studies from meta-analysis if they did not report behavioral outcomes of needle/syringe sharing combinable for data synthesis or if we could not calculate the study-specific effect size because of missing or insufficient data, in which case authors were contacted for additional information. For example, articles were excluded if between-group comparisons were based on HIV status instead of needle/syringe sharing behaviors, or sharing behaviors were defined as “ever shared needles/syringes.” Studies were not combinable in meta-analysis if NSP exposure was defined by study authors in a unique way (e.g., exchanging seven or more free needles/syringes per week from the program 16 ), the study measured syringe sharing as a continuous variable (e.g., mean number of needles/syringes exchanged in the past 30 days 17 ), or it was not possible to attribute intervention effects to NSP exposure (e.g., the outcome measure was ever shared needles/syringes 18 ). We reported findings from all studies not included in meta-analysis in the qualitative synthesis, organized by outcome category.
The search generated 4,513 citations from electronic databases and 76 citations by both hand-searching five journals and secondary searching ( Figure 1 ). After removing duplicates, 3,425 references were included for primary screening. We excluded 3,254 citations after examining their titles and abstracts. 171 full-text articles were retrieved to determine eligibility. Ultimately, 35 articles reporting on 42 interventions met the criteria for inclusion in this review and were included in the qualitative synthesis. 16 – 50
PRISMA flow diagram of the different phases of the systematic review.
Study characteristics
Table 1 presents summary characteristics of the 42 intervention effects (reported in 35 included articles) and descriptions of reported interventions related to NSP published between 1999 and 2021. 16 – 50 Two of these articles reported on Avahan, including community interventions for NSP in India, but presented different outcomes; 24 , 25 three other articles reported different outcomes and timepoints from the Cross-Border Project, a large serial cross-sectional study conducted in multiple sites in Vietnam and China. 22 , 26 , 27 The 42 intervention effects included 56,751 total participants; individual study sample sizes ranged from 105 to 20,640. Study designs included 21 cross-sectional studies, six serial cross-sectional studies, two quasi-experimental/non-randomized trials, two time-series studies, one before-after study, one cluster-randomized controlled trial, one prospective cohort study, and one retrospective case-control study. Most of the included articles were conducted in Asia (Bangladesh, China, India, Iran, Nepal, Pakistan, and Vietnam) and Russia. Several trial or cohort designs (one cluster randomized controlled trial, two group non-randomized controlled trials, and one prospective cohort study) were used to evaluate NSP programs in China. Three studies were conducted in Eastern Europe, including one time-series study in Georgia, one cross-sectional study in Croatia, and one cross-sectional study in Bulgaria. All included studies were from lower-middle and upper-middle-income countries; none were from countries classified as low-income by the World Bank. Study participants were predominantly male and young; ages ranged from 18–50 years old. Only one cross-sectional study, from Mexico, focused on female sex workers who injected drugs.
Description of included studies and reported NSP interventions
HCV: hepatitis C virus
IQR: interquartile range.
NGO: non-governmental organization.
NSP: needle and syringe program, or needle and syringe exchange program.
STI: sexually transmitted infection
Intervention characteristics varied across studies. Peer-driven and social networks were the most common methods to recruit people who inject drugs. Peer educators, non-governmental organizations, and outreach health workers usually partnered with the NSP intervention to facilitate on-site and outreach needle/syringe exchange. Secondary exchange by peers and pharmacy vouchers also served as sources of clean needles and syringes. Most participants were also exposed to other intervention activities, such as HIV information sessions, health education, distribution of free condoms and injection equipment, and voluntary HIV counseling and testing.
Table 2 presents a summary of comparative outcomes reported, including needle-sharing behaviors and HIV and other STI-related outcomes, by study. Overall, 21 interventions presented outcomes measured at the individual-level and 19 at the community-level. We could not determine level of outcome measurements in two interventions. Among 30 interventions that reported needle-sharing behavior outcomes, 15 did not specify the direction of sharing. One reported the outcome of lifetime/ever shared needles/syringes. The other 14 included descriptions of how needles/syringes were shared, which we used to assign receptive and distributive needle-sharing outcomes. The most frequent additional HIV-related outcomes reported were HIV incidence and HIV prevalence, while a few studies measured hepatitis B, hepatitis C, syphilis, and chlamydia prevalence.
Comparative outcomes reported by included studies a
Cell shade in light green: outcome reported; in dark green: outcome reported and included in meta-analysis.
Combined receptive and distributive outcomes per study, when both reported.
Shared injecting paraphernalia include containers, cooker, cotton, filter, rinse water, solutions, etc.
Other injection outcomes include return of needles, frontloading/backloading, needle sharing frequency, injection frequency, number of sharing partner, etc.
Other STIs include chlamydia, gonorrhea, HBV, HCV, and syphilis.
Outcome reported is HIV testing prior to the survey. Duration or time frame of the measurement is unknown.
Needle-sharing outcome reported is ever shared needles/syringes.
Unique categorical measure of exposure: accessibility of NSP services (low, middle, high).
Unique categorical measure of exposure: low or high NSP user.
Unique categorical measure of exposure: whether received 7 or more free needles/syringes.
Table 3 presents the risk of bias assessment for each intervention. Most interventions did not employ a cohort design (N=34) or perform randomization when enrolling participants and assigning interventions (N=40). Most interventions (N=32) were evaluated through cross-sectional or serial cross-sectional methods. Therefore, those study results are subject to limitations due to cross-sectional design, weak participant representativeness, and non-equivalence of comparison groups (if applicable). One prospective cohort study, two before-after studies and two time-series studies were rated low rigor because of non-randomized designs and low follow-up rates, which were all below 80%. Only one of two non-randomized trials had a follow-up rate higher than 80%. Only one randomized trial was included, which showed the greatest rigor. This cluster randomized controlled trial in China investigated a needle social marketing strategy at the community level among 823 young people who inject drugs from 2002 to 2003. 43 Although its findings suggested significant NSP intervention benefits, it did not randomly select participants for assessment and the comparison groups were not equivalent at baseline. 43 In fact, none of the included studies reported equivalent group characteristics at baseline.
Quality assessment of included articles.
Study outcomes included in meta-analysis.
Below, we present findings for needle-sharing, HIV incidence and prevalence, and other HIV-related outcomes.
Needle/syringe sharing
Overall, 25 articles measured the effects of NSP exposure on needle/syringe sharing behaviors. 16 – 21 , 25 – 27 , 29 – 34 , 38 , 39 , 43 – 50 Of these, 16 articles provided comparable outcome data and were included in meta-analysis; 13 presented additional outcomes that were analyzed qualitatively.
Meta-analysis
Sixteen articles presenting 20 interventions 19 , 20 , 25 , 27 , 29 , 31 – 34 , 38 , 39 , 43 – 47 were meta-analyzed, resulting in a total sample size of 11,075, with 6,024 people who inject drugs exposed to the intervention and 5,051 in the reference group. Meta-analysis results are summarized in Table 4 .
Summary of meta-analyses of needle-sharing outcomes.
Receptive, Distributive, and Any (non-directional) needle sharing combined per study, when any of the three were available for a study.
Non-directional needle sharing estimate reported.
Receptive and Distributive needle sharing combined per study, when both were reported.
For overall needle-sharing behaviors (combining receptive, distributive, and non-directional needle sharing), NSP exposure was associated with a statistically significant protective effect when assessed at both the individual- and community-level ( Figure 2 ; individual-level: odds ratio [OR]=0.25, 95% confidence interval [CI]=0.16–0.39, p <0.001, I 2 =86%, 8 trials, n=3,947; community-level: OR=0.39, CI=0.22–0.69, 12 trials, n=6,850, p =0.001, I 2 =95%). 19 , 20 , 25 , 27 , 29 , 31 – 34 , 38 , 39 , 43 – 47 However, there was high statistical heterogeneity, likely due to significant differences in outcome measurements, intervention modality, intervention duration, and exposure types.
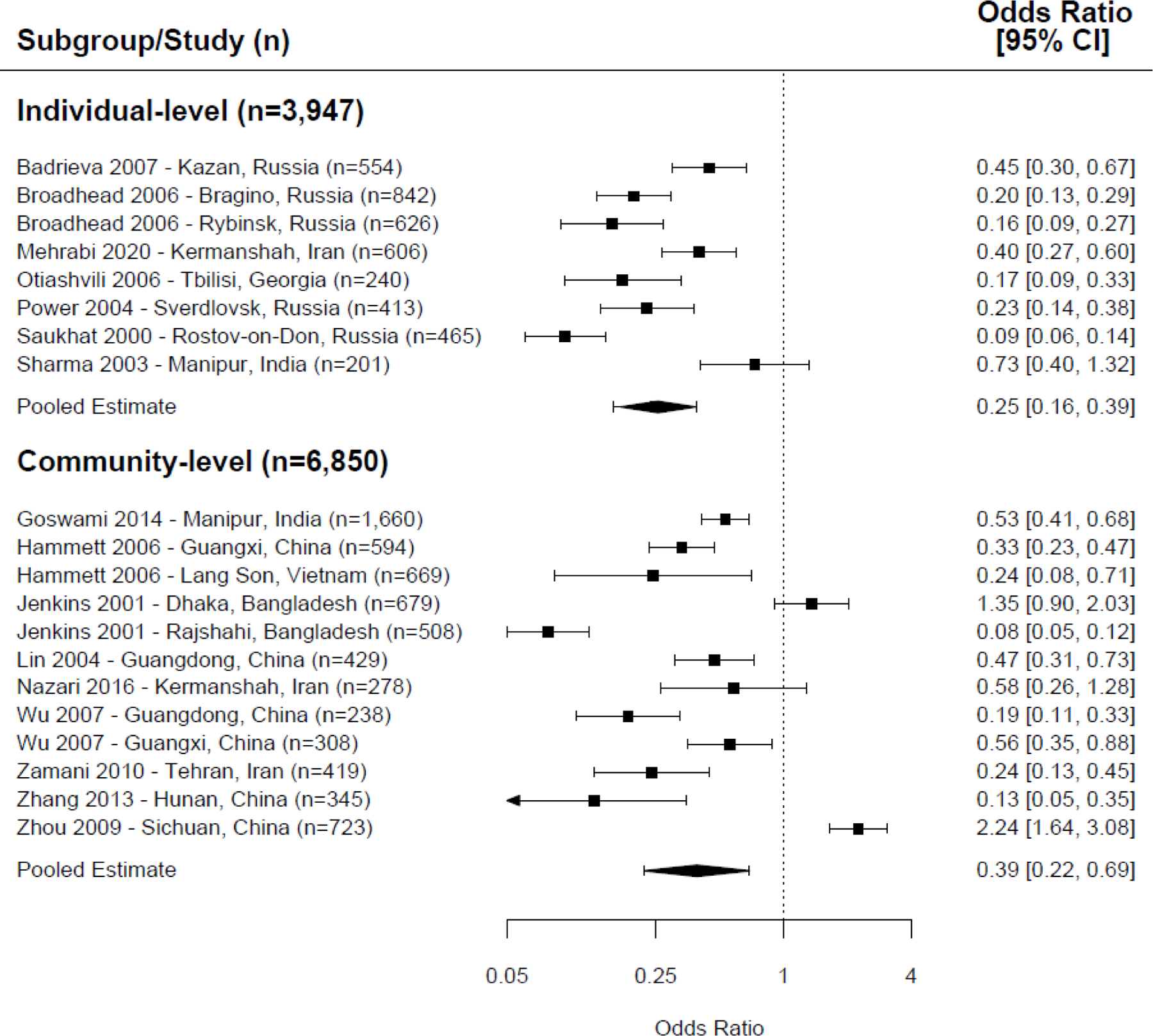
Meta-analysis of overall needle sharing (receptive, distributive, and non-directional needle sharing combined), exposure to NSP versus control/comparison group.
Separate analyses examined pooled effects of NSP exposure on receptive sharing and distributive sharing. For receptive sharing, we combined the effects of nine interventions in meta-analysis. We found that NSP exposure was associated with a lower odds of receptive sharing behaviors ( Figure 3 ) at both the individual-level (OR=0.39, CI=0.23–0.65, p <0.001, 3 trials, I 2 =70%) and the community-level (OR=0.30, CI=0.11–0.78, p =0.001, 6 trials, I 2 =95%). 19 , 27 , 29 , 32 , 34 , 39 , 44 Seven interventions examining the associations between NSP exposure and distributive sharing did not show a statistically significant association when combined in meta-analysis at either the individual- or community-level ( Figure 4 ; individual-level: OR=0.61, CI=0.31–1.21, p =0.16, 2 trials, I 2 =72%; community-level: OR=0.40, CI=0.14–1.15, p =0.09, 5 trials, I 2 =96%). 19 , 27 , 29 , 32 , 39
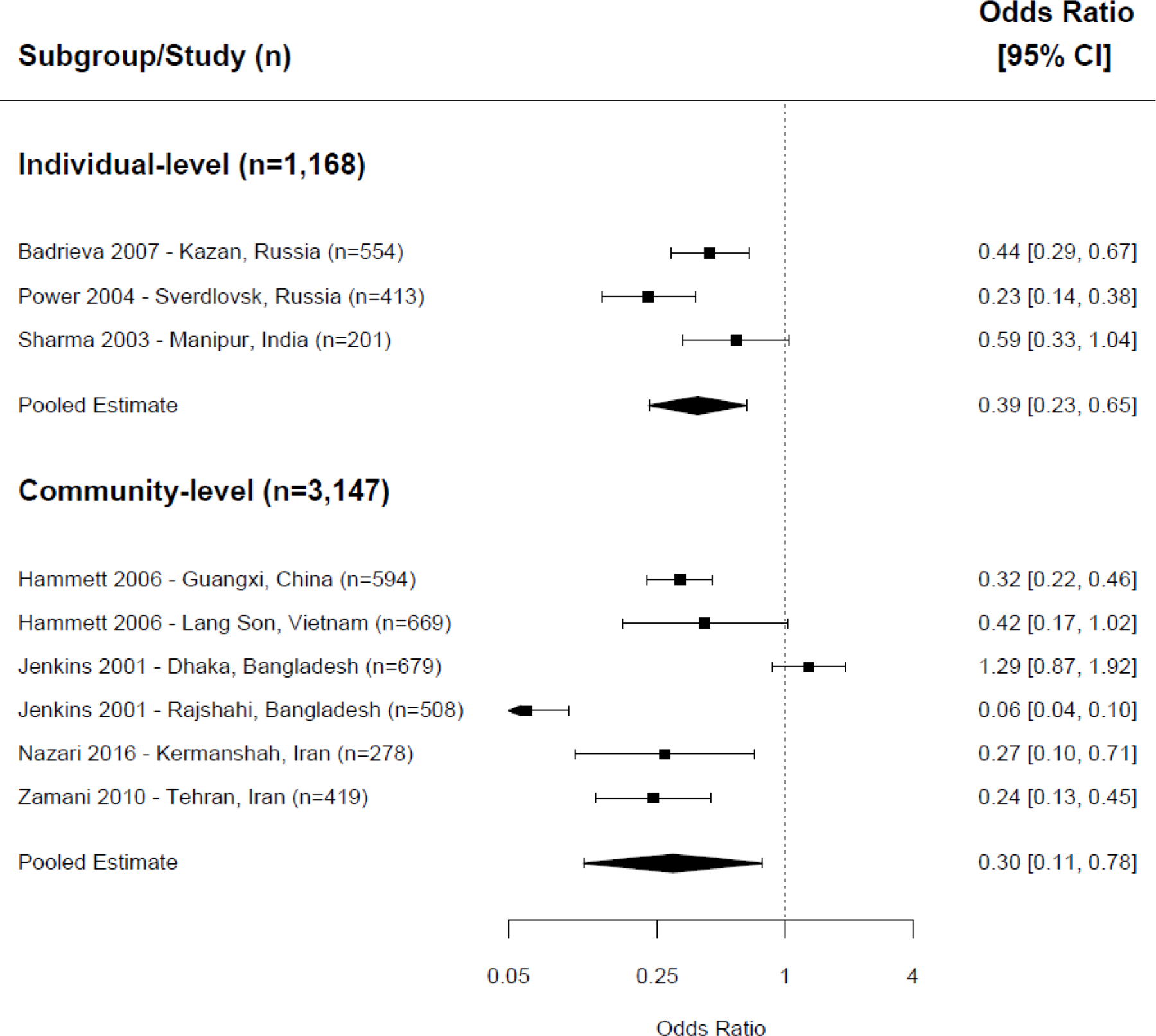
Meta-analysis of receptive needle sharing, exposure to NSP versus control/comparison group.
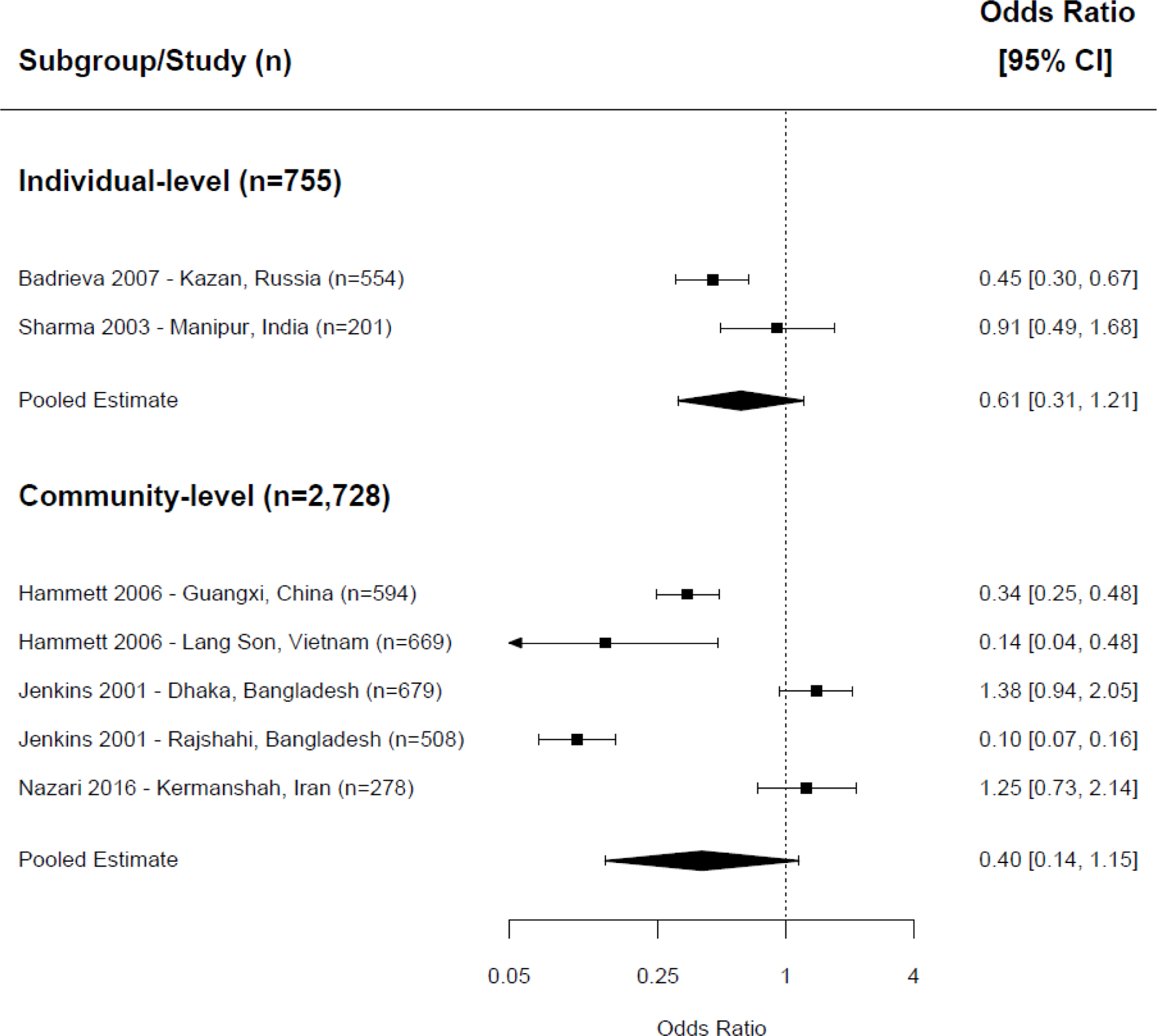
Meta-analysis of distributive needle sharing, exposure to NSP versus control/comparison group.
When we combined the effect sizes from studies that did not specify directionality of sharing ( Figure 5 ), 20 , 25 , 29 , 31 , 33 , 38 , 43 , 45 – 47 a protective association between exposure to NSP and needle/syringe sharing was observed among the five interventions at the individual-level (OR=0.18, CI=0.11–0.31, p <0.001, 5 trials, I 2 =83%). We also observed 58% lower odds of needle/syringe sharing at the community-level (OR=0.42, CI=0.19–0.91, p =0.027, 8 trials, I 2 =96%), although heterogeneity was significant.
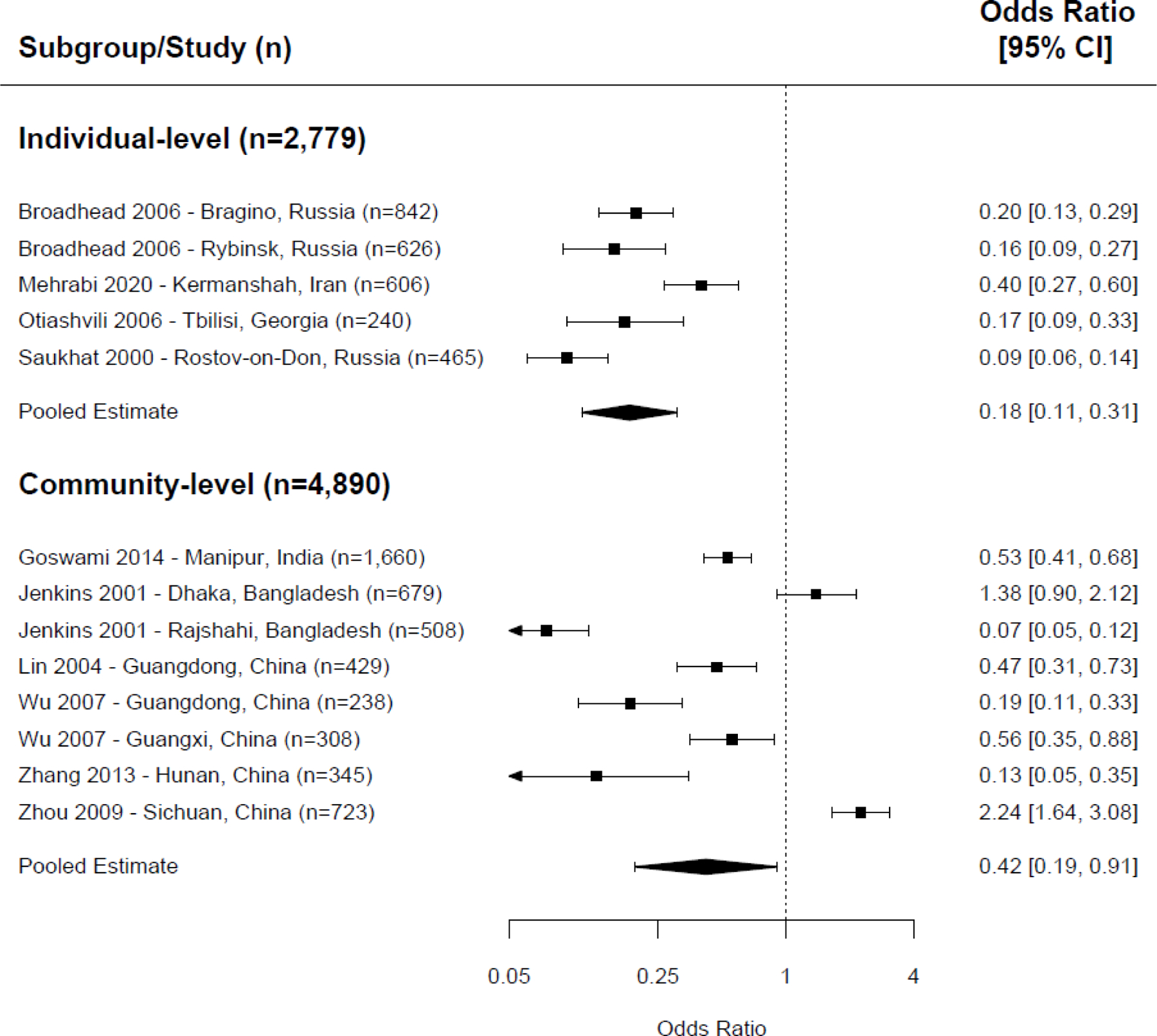
Meta-analysis of non-directional needle sharing, exposure to NSP versus control/comparison group.
In addition, six interventions reported both receptive and distributive sharing outcomes. 19 , 27 , 29 , 39 The pooled within-study effect of needle sharing was estimated per study, and we found statistically significant lower odds of this combined receptive and distributive needle/syringe sharing outcome among people who inject drugs who participated in NSP (OR=0.54, CI=0.34–0.87, p =0.01, 2 trials, I 2 =44%) with moderate heterogeneity at the individual-level. However, the protective association did not retain statistical significance when analyzed at the community-level (OR=0.31, CI=0.09–1.10, p =0.07, 4 trials, I 2 =97%).
Figure 6 shows a scatter plot of 20 intervention effect estimates (log odds ratio) measured in the meta-analysis against each study’s standard error. We observed a slightly asymmetric shape with more effect estimates from large studies scattered widely in a horizontal line at the middle top of the graph. The funnel plot suggests most included studies with similar standard errors reported protective effects of NSPs, potentially reflecting some reporting bias.
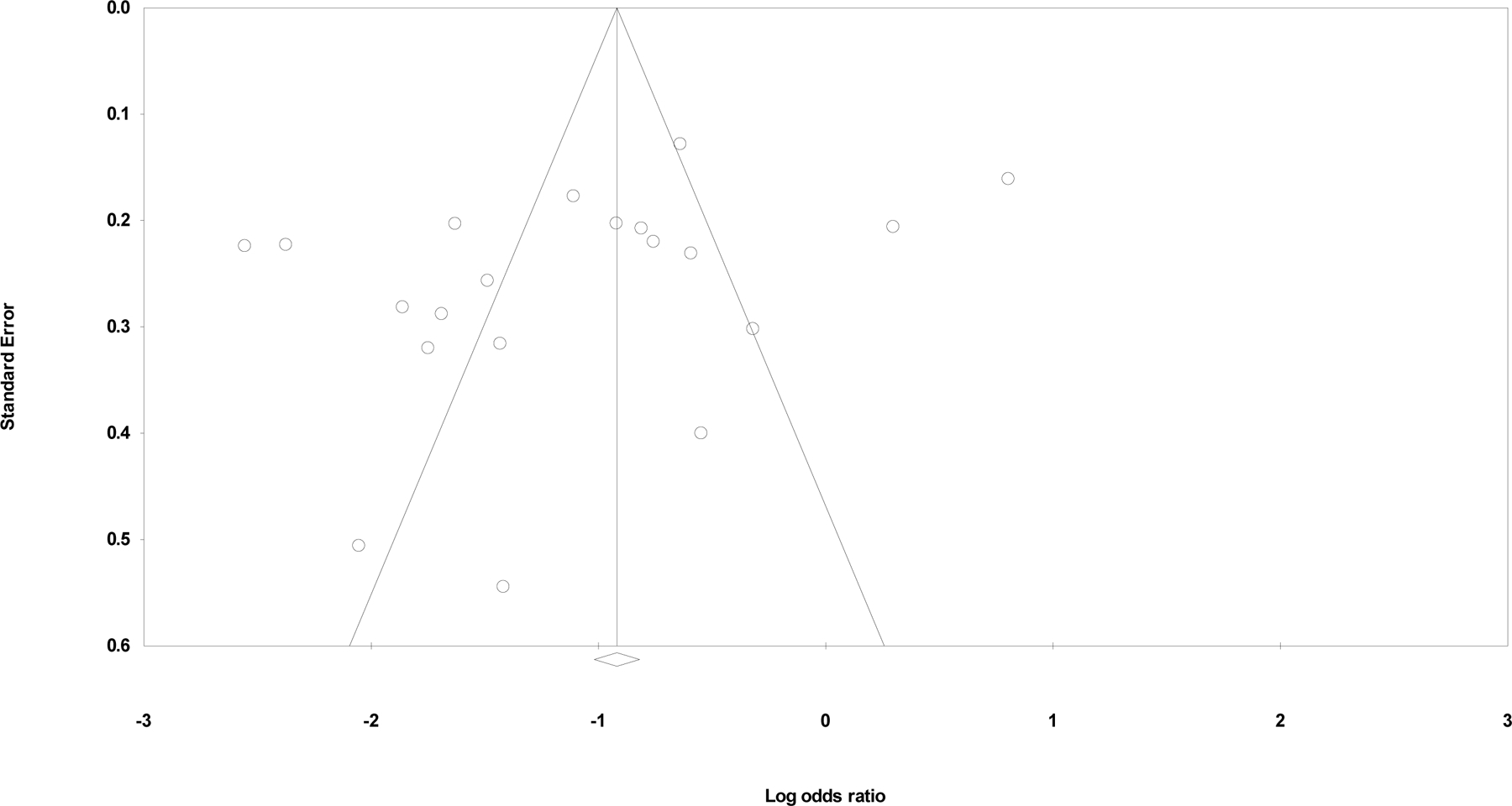
Funnel plot of standard error by log odds ratio, N=20.
Qualitative synthesis
In addition, 13 articles presented associations between NSP exposure and needle/syringe sharing related outcomes, but could not be included in meta-analysis as described in the methods section above. 16 – 21 , 31 , 32 , 34 , 39 , 48 – 50 Overall, six articles reported an association between NSP exposure and reduced needle/syringe sharing behaviors, although their definitions of NSP exposure varied widely. 16 , 17 , 21 , 48 – 50 Four cross-sectional studies in Iran published between 2004 and 2020 suggested that the odds of reporting needle/syringe sharing behaviors were lower among people who inject drugs who had high accessibility to NSP, or who received more sterile needles/syringes from NSP, or who attended NSP regularly, compared with their comparator groups. 16 , 48 – 50 Another cross-sectional study in 10 central and eastern European cities found statistically significant reductions in receptive needle/syringe sharing. 21 Less needle/syringe sharing was reported among NSP users in China 18 and Russia 17 as well. Other articles reported on sharing injection paraphernalia (cookers, filters, rinse water, solutions, containers, etc.) but effects of NSP varied widely – positive, neutral and negative – depending on specific study sites, comparators, and length of time from intervention. 16 , 17 , 19 – 21 , 31 , 32 , 34 , 39 , 48 , 49
HIV incidence and prevalence
HIV incidence was measured or estimated in 5 articles. 22 , 26 , 34 , 36 , 43 One cohort study in China found a statistically significant and substantial decrease in HIV incidence comparing participants in a cohort before NSP was available to a post-NSP cohort. 36 A second study from Russia presented government statistics on new HIV diagnoses (per 100,000 population) in the cities where NSPs were implemented, finding decreases in new diagnosis rate over the study period in one city (Verknaya Salda) and increases then decreases in new diagnoses in two other cities (Pervouralsk and Ekaterinburg). 34 The remaining studies calculated estimated measures of HIV incidence, based on the assumption that prevalent cases of HIV among new injectors were comparable to incidence. The strongest study design, a cluster-randomized trial in China, found that the NSP intervention was associated with reduced HIV incidence in one study site (Guangdong), but not the other (Guangxi); combined, the effect was not statistically significant. 43 One serial cross-sectional study, the Cross-Border Project, conducted in Vietnam and China reported estimated HIV incidence data in two articles. 22 , 26 Both articles found that estimated HIV incidence decreased substantially to low levels among new injectors by 24 months, although one article 22 found that estimated incidence continued to decline by 36 months while the other 26 saw some rebound after 36 months, particularly in one study site (Ning Ming, China). Four of these five articles also reported other behavioral or disease outcomes; generally, where HIV incidence decreased, sharing behaviors and other infections decreased as well.
Fifteen articles (20 interventions), mostly cross-sectional studies, reported associations between NSP and HIV prevalence. 18 , 22 , 23 , 25 – 27 , 30 , 31 , 35 , 37 , 38 , 40 – 43 Across these, we found no consistent association between NSP exposure and HIV prevalence.
Other HIV-related outcomes
Several studies reported positive changes in other HIV-related outcomes, such as significantly higher HIV testing uptake. 32 , 49 Additional studies showed inconsistent associations between NSP interventions and incidence or prevalence of other infectious diseases (chlamydia, gonorrhea, hepatitis B, hepatitis C, and syphilis). 25 , 28 , 31 , 36 , 41 , 43
We systematically reviewed the current evidence base for the effectiveness of NSP at both the individual- and community-level, with a focus on needle-sharing behaviors and other HIV-related outcomes. Although few rigorous trials were identified, we did find a relatively strong evidence base of mostly cross-sectional studies examining the association between NSPs and needle sharing, and a more limited evidence base for HIV incidence, HIV testing, and other infectious diseases.
In meta-analysis, NSPs were associated with fewer people who inject drugs receiving used needles/syringes at both the individual- and community-level. The effect of NSPs on distributive sharing was not statistically significant at either level. When we combined distributive and receptive needle/syringe sharing outcomes, we observed a more significant protective association for NSPs whose outcomes were measured at the individual-level than at the community-level. These findings are consistent with a direct benefit of NSPs for those who use them. However, this benefit seems to be primarily for receptive sharing – the impact of which is substantial enough that it can be seen even when measured at the community-level. Compared with other reviews exploring the effectiveness of NSP in reducing HIV incidence, 5 , 10 , 12 , 51 our approach to stratifying effects by level and directionality provides additional insight on specific risks. While the provision of clean, sterile needles/syringes is fundamental and necessary to reduce receptive sharing, it is not sufficient to reduce distributive sharing.
Beyond needle/syringe sharing, we also found that NSP exposure was associated with lower HIV incidence and higher HIV testing uptake. Findings on HIV prevalence and other infectious diseases were mixed; however, we attribute this to the cross-sectional nature of the study designs which could not assess causal relationships. Overall, findings from our review support the conclusion that NSPs are associated with better HIV outcomes.
We defined NSPs as programs both providing clean, sterile injecting equipment and also disposing of used injection equipment according to safety regulations. The included studies involved a variety of service delivery mechanisms, including pharmacy-based services and peer outreach. While several studies mentioned the cost of clean needles through pharmacies, not enough studies provided sufficient detail to analyze whether distribution modality made a difference in needle-sharing behaviors and other outcomes. However, programs which sell or otherwise provide needles/syringes to people who inject drugs through pharmacies have a number of benefits: they are relatively inexpensive to operate, are often open for long hours, are typically accessible in low-resourced settings including in rural areas, and may provide discretion to their clients. Though some research has already explored the benefits of pharmacy-based compared to facility-based NSP, 52 further work could examine the benefits of such programs.
In addition to understanding the individual- or community-level measurement and directionality of needle-sharing behaviors, it is also important to understand how NSPs influence needle disposal behaviors. Studies in high-income settings have posited that unsafe needle disposal outside of hospitals or other healthcare settings by people who inject drugs could increase risk of bloodborne infectious disease in the broader community; 53 , 54 NSPs could ameliorate this risk by reducing unsafe disposal behaviors. 55 However, none of the included articles reported the exchange rate of used needles/syringes or other unsafe disposal behaviors. No included articles provided details on whether all used needles/syringes were retrieved when study participants interacted with the NSP, or whether study participants were able to obtain new injection kits without having to return used needles/syringes. Future research could explore unsafe disposal behaviors in LMICs.
Because we included studies published from 1990 to 2021, we were able to qualitatively explore temporal changes in NSP implementation in countries like Bangladesh, China, India, Iran, Pakistan, Russia, and Vietnam which provided data from both the 1990s/2000s and more recently. Earlier NSP activities primarily focused on needle distribution and needle exchange, along with counselling and health education/promotion about drug use reduction and HIV prevention. Earlier programs tended to use non-governmental organizations or primary care workers, but more recent studies tended to involve peer educators as facilitators.
Participants in these included studies were predominantly male. While this may reflect local patterns of drug use in these particular LMIC settings, women who inject drugs are often underrepresented in the scientific and medical literature. 56 Previous research has found gender differences among people who inject drugs, in terms of HIV risk and needle-sharing behaviors. 2 , 57 Women who inject drugs have a higher likelihood of engaging in unsafe sexual practices and a compounded risk of contracting HIV. 58 The complex intersection of HIV and injection drug use could benefit from a socio-ecological approach and gender-specific interventions tailored to specific populations’ needs. For example, recruiting female outreach workers or female peer educators, coupled with women-focused behavioral interventions, has been found effective. 59
Despite heterogeneity in intervention modalities and outcome measures, we found evidence that NSP is associated with reduced receptive and overall needle-sharing behaviors in both individual-level and community-level NSP in LMICs. Interestingly, these behaviors which typically are only measured at the individual-level were strong enough to demonstrate community-level effects. NSPs appear to be an effective intervention component to prevent HIV and other blood-borne infections by improving access to sterile needles/syringes and discouraging people who inject drugs from sharing used injection equipment. There is also a literature gap in African, Latin American, and Caribbean countries where NSP implementation is less common, though HIV prevalence may be high.
NSPs have been promoted as an effective intervention to reduce HIV, hepatitis C, and hepatitis B transmission among people who inject drugs in high-income settings. 51 We identified several LMICs in Asia and Eastern Europe that implemented large-scale NSPs as part of harm reduction programs. According to the latest Global State of Harm Reduction report, although NSPs are officially allowed and scaling up in many Asian and Eastern European countries where our included studies were based, their service capacity is relatively limited because of weak political support and shrinking funds. 60 For example, illicit drug use is an indictable act in China. If arrested and found to have relapsed, people who inject drugs could be directed to receive compulsory methadone maintenance therapy or referred to mandatory detoxification centers. 45 Stringent drug-control regulations and police confinement appear to limit access to NSPs by people who inject drugs. Some Asian countries, including Laos, the Philippines, and Mongolia, still prohibit the implementation of NSPs even though NSPs have been shown as an effective harm-reduction approach in nearby countries based on the 2020 Harm Reduction International report. 60 Punitive drug policies, limited funding, and a high level of stigma also substantially limit the coverage and quality of NSPs in Eastern European countries. 60 , 61 For example, Bulgaria suspended a NSP site in July 2020 due to unstable funding after one year of reopening operation. 62 Punitive and stringent regulations around injection drug use also make people who inject drugs seeking NSPs in Russia and Ukraine at high risk of discrimination, police hostility, and arrest, 60 which could be further exacerbated by the current conflict. Therefore, adapting NSPs to local policies and maintaining NSPs’ accessibility in LMICs deserve further investigation.
This review has several strengths. We explored both individual- and community-level outcome measurements and included different types of needle/syringe-sharing behaviors. Use of an eight-item risk of bias tool allowed us to assess the rigor of included studies. By not restricting inclusion by language and searching multiple databases, we minimized the possibility of missing relevant studies. We also synthesized the most recent empirical evidence of NSP’s impact on HIV-related outcomes through July 2021. Finally, we tried to achieve quality assurance by having two independent study reviewers/assistants screen articles, abstract data, and resolve any discrepancies.
However, our study also has several limitations. First, our search strategy was focused on HIV-related outcomes; we therefore may have missed other studies examining the impact of NSPs on needle/syringe sharing behaviors without mentioning HIV, or that focused exclusively on hepatitis C or other outcomes. Second, most of the included studies were cross-sectional in design and did not provide data adjusted for confounders, which limits the inferences we can draw. Further, there was significant heterogeneity across studies in intervention length/frequency, recruitment strategy, choice of facilitators/implementers, delivery methods, and other concurrent intervention activities. Due to the limited number of studies for each outcome, we were also unable to conduct meta-regression to examine study-level factors associated with positive outcomes. Future studies could consider evaluating NSP designs or procedures in difference settings and regions. How to customize program designs and intervention activities considering local patterns of drug use, population characteristics, and regulations to enhance NSP’s positive impacts is worth further academic attention. Third, we were unable to include several effect measures in meta-analysis due to insufficient information, and we failed to obtain responses from correspondent authors after email inquiries. This reduced the number of included interventions for the meta-analysis. Fourth, there were no standardized measurements for NSP exposure, and most studies relied on participants’ self-reported behavioral outcomes. Due to the nature of the intervention, convenience and purposive sampling methods were also the most used recruitment methods. Therefore, the pooled estimates of NSP impacts are likely affected by measurement bias, social-desirability bias, and selection bias. Finally, implementation science research in both high- and low-resource settings has generally shifted from focusing on NSP to providing “comprehensive HIV prevention and care” including NSP, opioid agonist treatment, methadone maintenance treatment, and antiretroviral therapy for people who inject drugs; 63 two recent studies of such comprehensive programs in LMICs (HPTN 074 in Eastern Europe and Southeast Asia, 64 the DRIVE study in Vietnam 65 ) have found very low HIV incidence among people who inject drugs. Better understanding how NSPs contribute to the overall success of such comprehensive packages is challenging but worth future investigation.
In conclusion, we examined the association of NSPs with HIV-related outcomes in LMICs over the past three decades. We found that exposure to NSP across studies was overall associated with reduced needle/syringe sharing and reduced HIV incidence among people who inject drugs. The meta-analysis also found that NSPs showed a statistically significant beneficial association with reducing receptive sharing, non-directional sharing, and overall needle-sharing behaviors among people who inject drugs when measured at the individual-level. However, we did not find a statistically significant protective association with distributive needle-sharing behaviors at either the individual- or community-level.
Acknowledgements:
The authors wish to thank Elizabeth Jere, Carolyn Pleisca, Sarah Mauch, Alison Groves, Rachel Hower, Devaki Nambiar, Jennifer Gonyea, Andrea Ippel, Kirk Fiereck, Prossy Namusisi, and Indira Prihartono for their coding work on this project, Lindsay Cooper for her assistance in checking the Spanish language abstract, and Elena Tuerk and Julie Denison for their contributions to study development and supervision.
This work was supported by the US National Institute of Mental Health (grant numbers R01MH071204, R01MH090173, R01MH125798).
Appendix A. Search strategy for all databases, last searched on July 25, 2021
(“needle exchange” OR “needle distribution” OR “needle sales” OR “syringe sales” OR “syringe distribution” OR “syringe exchange” OR “syringe-exchange” OR “needle-exchange” OR “needle syringe programs” OR “needle syringe program” OR “needle syringe programme” OR “needle exchange programmes”) AND (HIV or AIDS)
Sociological Abstracts
(‘needle exchange’ OR ‘needle distribution’ OR ‘needle sales’ OR ‘syringe sales’ OR ‘syringe distribution’ OR ‘syringe exchange’ OR ‘syringe-exchange’ OR ‘needle-exchange’ OR ‘needle syringe programs’ OR ‘needle syringe program’ OR ‘needle syringe programme’ OR ‘needle exchange programmes’) AND (HIV or AIDS)
Hand search of the following journals:
AIDS and Behavior
AIDS Education and Prevention
the International Journal of Drug Policy
Conflicts of interest: The authors have no conflicts of interest to declare.
Declarations
Ethics approval: Because this is a systematic review of published data, no ethical approval was required.
Consent to participate: Not applicable
Consent for publication: Not applicable
Code availability: Not applicable
Registration: Not registered on PROSPERO.
Availability of data and material
Available upon request to the corresponding author.
- 1. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. The Lancet Global Health 2017; 5(12): e1192–e207. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Strathdee SA, Galai N, Safaiean M, et al. Sex differences in risk factors for hiv seroconversion among injection drug users: a 10-year perspective. Arch Intern Med 2001; 161(10): 1281–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Mesquita F, Jacka D, Ricard D, et al. Accelerating harm reduction interventions to confront the HIV epidemic in the Western Pacific and Asia: the role of WHO (WPRO). Harm reduction journal 2008; 5: 26. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Des Jarlais DC, Kerr T, Carrieri P, Feelemyer J, Arasteh K. HIV Infection among Persons who inject Drugs: Ending Old Epidemics and Addressing New Outbreaks. AIDS (London, England) 2016; 30(6): 815–26. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe Programmes in people who inject drugs – An overview of systematic reviews. BMC public health 2017; 17. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Des Jarlais DC, Pinkerton S, Hagan H, et al. 30 Years on Selected Issues in the Prevention of HIV among Persons Who Inject Drugs. Advances in Preventive Medicine 2013; 2013: 346372. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. van den Hoek JA, van Haastrecht HJ, Coutinho RA. Risk reduction among intravenous drug users in Amsterdam under the influence of AIDS. Am J Public Health 1989; 79(10): 1355–7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Kral AH, Bluthenthal RN. What is it about needle and syringe programmes that make them effective for preventing HIV transmission? International Journal of Drug Policy 2003; 14(5): 361–3. [ Google Scholar ]
- 9. Abdul-Quader AS, Feelemyer J, Modi S, et al. Effectiveness of Structural-Level Needle/Syringe Programs to Reduce HCV and HIV Infection Among People Who Inject Drugs: A Systematic Review. AIDS and behavior 2013; 17(9): 2878–92. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. International journal of epidemiology 2014; 43(1): 235–48. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Salter ML, Go VF, Minh NL, et al. Influence of Perceived Secondary Stigma and Family on the Response to HIV Infection Among Injection Drug Users in Vietnam. AIDS Education and Prevention 2010; 22(6): 558–70. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Des Jarlais DC, Feelemyer JP, Modi SN, Abdul-Quader A, Hagan H. High coverage needle/syringe programs for people who inject drugs in low and middle income countries: a systematic review. BMC public health 2013; 13: 53. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic Reviews 2021; 10(1): 89. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. World Bank. World Bank Country and Lending Groups 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed June 1 2020).
- 15. Kennedy CE, Fonner VA, Armstrong KA, et al. The Evidence Project risk of bias tool: assessing study rigor for both randomized and non-randomized intervention studies. Systematic Reviews 2019; 8(1): 3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Vazirian M, Nassirimanesh B, Zamani S, et al. Needle and syringe sharing practices of injecting drug users participating in an outreach HIV prevention program in Tehran, Iran: a cross-sectional study. Harm reduction journal 2005; 2: 19. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Sergeyev B, Oparina T, Rumyantseva TP, et al. HIV Prevention in Yaroslavl, Russia: A Peer-Driven Intervention and Needle Exchange. Journal of Drug Issues 1999; 29(4): 777–803. [ Google Scholar ]
- 18. Luo W, Wu Z, Poundstone K, et al. Needle and syringe exchange programmes and prevalence of HIV infection among intravenous drug users in China. Addiction (Abingdon, England) 2015; 110(Suppl 1): 61–7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Badrieva L, Karchevsky E, Irwin KS, Heimer R. Lower injection-related HIV-1 risk associated with participation in a harm reduction program in Kazan, Russia. AIDS Educ Prev 2007; 19(1): 13–23. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Broadhead RS, Ryabkova M, Borch C, et al. Peer-driven HIV interventions for drug injectors in Russia: First year impact results of a field experiment. International Journal of Drug Policy 2006; 17(5): 379–92. [ Google Scholar ]
- 21. Des Jarlais DC, Friedmann P, Grund J-P, et al. HIV risk behaviour among participants of syringe exchange programmes in central/eastern Europe and Russia. International Journal of Drug Policy 2002; 13(3): 165–70. [ Google Scholar ]
- 22. Des Jarlais DC, Kling R, Hammett TM, et al. Reducing HIV infection among new injecting drug users in the China-Vietnam Cross Border Project. AIDS 2007; 21 Suppl 8: S109–14. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Eicher AD, Crofts N, Benjamin S, Deutschmann P, Rodger AJ. A certain fate: spread of HIV among young injecting drug users in Manipur, north-east India. AIDS Care 2000; 12(4): 497–504. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Ganju D, Ramesh S, Saggurti N. Factors associated with HIV testing among male injecting drug users: findings from a cross-sectional behavioural and biological survey in Manipur and Nagaland, India. Harm reduction journal 2016; 13(1): 21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Goswami P, Medhi GK, Armstrong G, et al. An assessment of an HIV prevention intervention among People Who Inject Drugs in the states of Manipur and Nagaland, India. International Journal of Drug Policy 2014; 25(5): 853–64. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Hammett TM, Des Jarlais DC, Kling R, et al. Controlling HIV epidemics among injection drug users: Eight years of cross-border HIV prevention interventions in Vietnam and China. PLoS ONE 2012; 7(8): e43141. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Hammett TM, Kling R, Johnston P, et al. Patterns of HIV prevalence and HIV risk behaviors among injection drug users prior to and 24 months following implementation of cross-border HIV prevention interventions in northern Vietnam and southern China. AIDS Educ Prev 2006; 18(2): 97–115. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Handanagic S, Sevic S, Barbaric J, et al. Correlates of anti-hepatitis C positivity and use of harm reduction services among people who inject drugs in two cities in Croatia. Drug and alcohol dependence 2017; 171: 132–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Jenkins C, Rahman H, Saidel T, Jana S, Hussain AMZ. Measuring the impact of needle exchange programs among injecting drug users through the National Behavioural Surveillance in Bangladesh. AIDS Education and Prevention 2001; 13(5): 452–61. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Khan AA, Khan A. Performance and coverage of HIV interventions for injection drug users: Insights from triangulation of programme, field and surveillance data from Pakistan. International Journal of Drug Policy 2011; 22(3): 219–25. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Lin P, Fan ZF, Yang F, et al. [Evaluation of a pilot study on needle and syringe exchange program among injecting drug users in a community in Guangdong, China]. Zhonghua Yu Fang Yi Xue Za Zhi 2004; 38(5): 305–8. [ PubMed ] [ Google Scholar ]
- 32. Nazari SSH, Noroozi M, Soori H, et al. The effect of on-site and outreach-based needle and syringe programs in people who inject drugs in Kermanshah, Iran. International Journal of Drug Policy 2016; 27: 127–31. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Otiashvili D, Gambashidze N, Kapanadze E, Lomidze G, Usharidze D. Effectiveness of needle/syringe exchange program in Tbilisi. Georgian Med News 2006; (140): 62–5. [ PubMed ] [ Google Scholar ]
- 34. Power R, Khalfin R, Nozhkina N, Kanarsky I. An evaluation of harm reduction interventions targeting injecting drug users in Sverdlovsk Oblast, Russia. International Journal of Drug Policy 2004; 15(4): 305–10. [ Google Scholar ]
- 35. Reynolds A The impact of limited needle and syringe availability programmes on HIV transmission-a case study in Kathmandu. Int J Drug Policy 2000; 11(6): 377–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Ruan Y, Liang S, Zhu J, et al. Evaluation of harm reduction programs on seroincidence of HIV, hepatitis B and C, and syphilis among intravenous drug users in southwest China. Sex Transm Dis 2013; 40(4): 323–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Samo RN, Altaf A, Memon A, Shah SA. Determinants of HIV sero-conversion among male injection drug users enrolled in a needle exchange programme at Karachi, Pakistan. Journal of the Pakistan Medical Association 2013; 63(1): 90–4. [ PubMed ] [ Google Scholar ]
- 38. Saukhat SR, Vorontsov DV, Tormozova NM, et al. [The effect of a syringe-exchange program on a decrease in the risk of HIV infection among intravenous narcotic abusers in the city of Rostov-on-Don]. Zh Mikrobiol Epidemiol Immunobiol 2000; (4): 89–92. [ PubMed ]
- 39. Sharma M, Panda S, Sharma U, Singh HN, Sharma C, Singh RR. Five years of needle syringe exchange in Manipur, India: programme and contextual issues. International Journal of Drug Policy 2003; 14(5–6): 407–15. [ Google Scholar ]
- 40. Strathdee SA, Lozada R, Martinez G, et al. Social and structural factors associated with HIV infection among female sex workers who inject drugs in the Mexico-US border region. PLoS One 2011; 6(4): e19048. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Vassilev ZP, Hagan H, Lyubenova A, et al. Needle exchange use, sexual risk behaviour, and the prevalence of HIV, hepatitis B virus, and hepatitis C virus infections among Bulgarian injection drug users. International Journal of STD and AIDS 2006; 17(9): 621–6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Wu Q, Kamphuis C, Duo L, Luo J, Chen Y, Richardus JH. Coverage of harm reduction services and HIV infection: a multilevel analysis of five Chinese cities. Harm reduction journal 2017; 14(1): 10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Wu Z, Luo W, Sullivan SG, et al. Evaluation of a needle social marketing strategy to control HIV among injecting drug users in China. AIDS 2007; 21 Suppl 8: S115–22. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Zamani S, Vazirian M, Nassirimanesh B, et al. Needle and syringe sharing practices among injecting drug users in Tehran: a comparison of two neighborhoods, one with and one without a needle and syringe program. AIDS and behavior 2010; 14(4): 885–90. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Zhang L, Chen X, Zheng J, et al. Ability to access community-based needle-syringe programs and injecting behaviors among drug users: A cross-sectional study in Hunan Province, China. Harm reduction journal 2013; 10(1). [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Zhou JS, Zhang KL, Zhang LL, et al. A quasi-experimental study on a community-based behaviour change programme among injecting drug users in Sichuan, China. Int J STD AIDS 2009; 20(2): 125–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Mehrabi Y, Etemad K, Noroozi A, et al. Correlates of injecting paraphernalia sharing among male drug injectors in Kermanshah, Iran: implications for HCV prevention. Journal of Substance Use 2020; 25(3): 330–5. [ Google Scholar ]
- 48. Naserirad M, Beulaygue IC. Accessibility of Needle and Syringe Programs and Injecting and Sharing Risk Behaviors in High Hepatitis C Virus Prevalence Settings. Subst Use Misuse 2020; 55(6): 900–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Noroozi M, Marshall BDL, Noroozi A, et al. Do needle and syringe programs reduce risky behaviours among people who inject drugs in Kermanshah City, Iran? A coarsened exact matching approach. Drug Alcohol Rev 2018; 37 Suppl 1: S303–S8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Noroozi M, Noroozi A, Sharifi H, et al. Needle and Syringe Programs and HIV-Related Risk Behaviors Among Men Who Inject Drugs: A Multilevel Analysis of Two Cities in Iran. International journal of behavioral medicine 2019; 26(1): 50–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Sawangjit R, Khan TM, Chaiyakunapruk N. Effectiveness of pharmacy-based needle/syringe exchange programme for people who inject drugs: a systematic review and meta-analysis. Addiction (Abingdon, England) 2017; 112(2): 236–47. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Vorobjov S, Uusküla A, Abel-Ollo K, Talu A, Rüütel K, Des Jarlais DC. Comparison of injecting drug users who obtain syringes from pharmacies and syringe exchange programs in Tallinn, Estonia. Harm reduction journal 2009; 6: 3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Burris S, Welsh J, Ng M, Li M, Ditzler A. State Syringe and Drug Possession Laws Potentially Influencing Safe Syringe Disposal by Injection Drug Users. Journal of the American Pharmaceutical Association (1996) 2002; 42(6, Supplement 2): S94–S8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Golub ET, Bareta JC, Mehta SH, McCall LD, Vlahov D, Strathdee SA. Correlates of Unsafe Syringe Acquisition and Disposal Among Injection Drug Users in Baltimore, Maryland. Substance Use & Misuse 2005; 40(12): 1751–64. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Bluthenthal RN, Anderson R, Flynn NM, Kral AH. Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug and alcohol dependence 2007; 89(2): 214–22. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. El-Bassel N, Strathdee SA. Women who use or inject drugs: an action agenda for women-specific, multilevel and combination HIV prevention and research. Journal of acquired immune deficiency syndromes (1999) 2015; 69(Suppl 2): S182–S90. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Tuchman E Women’s injection drug practices in their own words: a qualitative study. Harm reduction journal 2015; 12(1): 6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Evans JL, Hahn JA, Page-Shafer K, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO study). Journal of Urban Health 2003; 80(1): 137–46. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Wechsberg WM, Deren S, Myers B, et al. Gender-specific HIV prevention interventions for women who use alcohol and other drugs: The evolution of the science and future directions. Journal of acquired immune deficiency syndromes (1999) 2015; 69(0 1): S128–S39. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Harm Reduction International. Global State of Harm Reduction 2020 2020. https://www.hri.global/files/2020/10/26/Global_State_HRI_2020_BOOK_FA.pdf (accessed November 17 2020).
- 61. Lancet The. The future of harm reduction programmes in Russia. The Lancet 2009; 374(9697): 1213. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Péter S, Georgieva Y. The Oldest Harm Reduction Organisation in Bulgaria Shut Down 2020. https://drogriporter.hu/en/the-oldest-harm-reduction-organisation-in-bulgaria-shut-down/ (accessed November 17 2020).
- 63. World Health Organization, United Nations Office of Drugs and Crime, Joint United Nations Programme on HIV/AIDS. WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users Geneva, Switzerland: WHO, 2009. [ Google Scholar ]
- 64. Miller WC, Hoffman IF, Hanscom BS, et al. A scalable, integrated intervention to engage people who inject drugs in HIV care and medication-assisted treatment (HPTN 074): a randomised, controlled phase 3 feasibility and efficacy study. Lancet 2018; 392(10149): 747–59. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Des Jarlais DC, Huong DT, Oanh KTH, et al. Ending an HIV epidemic among persons who inject drugs in a middle-income country: extremely low HIV incidence among persons who inject drugs in Hai Phong, Viet Nam. Aids 2020; 34(15): 2305–11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
- View on publisher site
- PDF (1.8 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Research article
- Open access
- Published: 11 April 2017
Effectiveness of needle and syringe Programmes in people who inject drugs – An overview of systematic reviews
- Ricardo M Fernandes 1 , 3 ,
- Maria Cary 2 ,
- Gonçalo Duarte 1 ,
- Gonçalo Jesus 1 ,
- Joana Alarcão 1 ,
- Carla Torre 2 ,
- Suzete Costa 2 ,
- João Costa 1 , 3 &
- António Vaz Carneiro 1 , 3
BMC Public Health volume 17 , Article number: 309 ( 2017 ) Cite this article
28k Accesses
166 Citations
83 Altmetric
Metrics details
Needle and syringe programmes (NSP) are a critical component of harm reduction interventions among people who inject drugs (PWID). Our primary objective was to summarize the evidence on the effectiveness of NSP for PWID in reducing blood-borne infection transmission and injecting risk behaviours (IRB).
We conducted an overview of systematic reviews that included PWID (excluding prisons and consumption rooms), addressed community-based NSP, and provided estimates of the effect regarding incidence/prevalence of Human Immunodeficiency Virus (HIV), Hepatitis C virus (HCV), Hepatitis B virus (HBV) and bacteremia/sepsis, and/or measures of IRB. Systematic literature searches were undertaken on relevant databases, including EMBASE, MEDLINE, and PsychINFO (up to May 2015). For each review we identified relevant studies and extracted data on methods, and findings, including risk of bias and quality of evidence assessed by review authors. We evaluated the risk of bias of each systematic review using the ROBIS tool. We categorized reviews by reported outcomes and use of meta-analysis; no additional statistical analysis was performed.
We included thirteen systematic reviews with 133 relevant unique studies published between 1989 and 2012. Reported outcomes related to HIV ( n = 9), HCV ( n = 8) and IRB ( n = 6). Methods used varied at all levels of design and conduct, with four reviews performing meta-analysis. Only two reviews were considered to have low risk of bias using the ROBIS tool, and most included studies were evaluated as having low methodological quality by review authors. We found that NSP was effective in reducing HIV transmission and IRB among PWID, while there were mixed results regarding a reduction of HCV infection. Full harm reduction interventions provided at structural level and in multi-component programmes, as well as high level of coverage, were more beneficial.
Conclusions
The heterogeneity and the overall low quality of evidence highlights the need for future community-level studies of adequate design to support these results.
Trial registration
The protocol of this systematic review was registered in Prospective Register of Systematic Reviews (PROSPERO 2015: CRD42015026145 ).
Peer Review reports
People who inject drugs (PWID) experience high levels of morbidity and mortality. Drug-related harms include overdose, drug-related deaths, and blood-borne infections such as Human Immunodeficiency Virus (HIV), Hepatitis C (HCV), Hepatitis B (HBV), and bacteremia/sepsis. HCV is currently the most prevalent infectious disease affecting PWID, while HIV prevalence rates are lower. In an estimated total of 12.7 to 16 million PWID worldwide, it is believed that 1.2 million are infected with HIV [ 1 ] and 10 million are infected with HCV [ 2 ]. Sharing needles and syringes, as well as other injecting paraphernalia, is a key route of transmission of these infections [ 3 ].
Needle and syringe programmes (NSP) are thought to be a critical component of harm reduction interventions among PWID [ 3 , 4 ]. The first NSP was established in the 1980s [ 5 ], in response to the global HIV epidemic, with the goal of providing access and encouraging the use of sterile injection paraphernalia by PWID. Since then, provision of these services has grown rapidly. Importantly, a shift in paradigm has favoured NSP as components of harm reduction or harm minimization policies, which focus on reducing all drug-related harms, i.e. preventing HIV, HBV and HCV infection, minimizing needle and syringe sharing and reuse, reducing the volume of discarded needles and syringes in the environment, and facilitating access to sterile paraphernalia. Furthermore, NSP may also promote the use of condoms and provide opportunistic relevant health information and services [ 3 ].
NSP are complex health interventions with several interacting components, such as behavioural changes in PWID and providers, a complex operating framework (users, providers, setting, health systems), and some degree of flexibility of interventions [ 6 ]. There is considerable variability among regions and countries in service provision, coverage and range of harm reduction interventions offered by NSP. In particular, a variety of measures have been developed to improve access to and use of sterile injecting equipment and to increase users’ choice. These include several methods of distribution or sale such as conventional NSP in fixed-sites, pharmacy-based distribution, dispensing machines and outreach programmes – often using a mobile van or bus, or through home-visits [ 3 ]. Generally, pharmacy and specialist needle exchange provides a wide range of harm reduction information and advice, along with clean needles and syringes and possibly injecting paraphernalia. The legal framework in which NSP operate also varies at country level [ 7 ]. Pharmacy-based NSP are known to be in place in at least Australia [ 8 ], Belgium, France, Ireland, Kyrgyzstan, the Netherlands, Portugal, Spain, Slovenia, Ukraine [ 7 , 9 ], UK [ 10 ], and New Zealand [ 11 ].
Many systematic reviews on the effectiveness of interventions providing injecting equipment have been conducted to date. Overviews of reviews which compile information from these multiple systematic reviews, have also been published. The aim of these overviews is to provide end-users with a comprehensive and critical summary of the available evidence on this intervention. Overviews are of particular interest in this field because existing systematic reviews have focused on disparate questions, regarding either specific populations (e.g. country-specific evidence), specific types of interventions (e.g. different types of NSP provision) or, most commonly, specific outcomes (e.g. HIV or HCV transmission) [ 12 – 14 ]. Furthermore, there is variability in methods used in these systematic reviews, including the assessment of possible biases and the use of quantitative synthesis (meta-analysis). Nevertheless, to the best of our knowledge, the published overviews in this field have focused only on specific outcomes, i.e. transmission of HIV and/or HCV.
Our primary objective was to conduct an overview of systematic reviews that evaluated the evidence of the effectiveness of NSP for PWID across a range of different relevant outcomes, i.e. blood-borne infection transmission and injecting risk behaviours (IRB).
Our secondary objective was to assess how different aspects of NSP provision, including provider, setting, coverage and any related component delivered in parallel, such as harm reduction services and opiate substitution therapy, modified the effect of NSP, with a particular focus on pharmacy-driven NSP.
We followed current guidance on the conduct of overviews of reviews, including recommendations from the Cochrane Collaboration [ 15 ] and guidance on public health intervention reviews by the Centre for Reviews and Dissemination of the University of York [ 16 ]. We also followed the recommendations from the PRISMA-P statement regarding reporting items that we considered applicable to this overview [ 17 ]. The protocol for this overview was registered at PROSPERO (CRD42015026145) available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015026145 .
Eligibility criteria
For inclusion in this overview, studies had to meet the following criteria:
Study design: systematic reviews, operationally defined as studies reporting a clearly stated set of objectives, eligibility criteria, a systematic search using two or more sources, a systematic presentation of the characteristics and findings of the included studies, and estimates of the size and direction of the effect of interventions presented as numerical data, on an individual study-level basis and/or with quantitative synthesis (meta-analysis);
Participants: PWID, defined as people who inject some form of drug at the beginning of the study. We excluded studies focusing exclusively on participants whose consumption was confined to prisons and consumption rooms, since these are populations with distinct characteristics from our target population;
Interventions: systematic reviews had to evaluate community-based NSP, defined as the supply of at least needles and syringes, with or without other injecting paraphernalia for the preparation and consumption of drugs;
Outcomes: required reported outcomes included the incidence and/or prevalence of blood-borne infections (HIV, HCV, HBV and bacteremia/sepsis), and/or measures of IRB (including but not limited to syringe re-use, borrowing, sharing, renting and lending).
When more than one review included exactly the same studies, the review that reported the most complete presentation of results was selected for inclusion in the overview.
Search strategy and screening
Searches were undertaken on MEDLINE®In-Process & Other Non-Indexed Citations (from 1946 to 12th of May 2015), EMBASE (from 1974 to 12th of May 2015) and PsycINFO (from 1806 to 12th of May 2015) via the OVID SP interface. In addition, the following databases were searched: the NHS Economic Evaluation Database (NHS EED), the Database of Abstracts of Reviews of Effects (DARE), the Cochrane Database of Systematic Reviews (CDSR), the National Institute for Health and Clinical Excellence (NICE), the Campbell Library of Systematic Reviews and the Database of Promoting Health Effectiveness Reviews (DoPHER). The search strategies for each database are shown in a supplementary file (Additional file 1 ). No language or other types of restrictions were applied. In addition to the database searches, handsearching of the references of the included reviews was undertaken to identify further relevant studies.
Titles and abstracts of articles identified were screened independently by two authors (MC, JA), and classified as include, unclear or exclude. The full reports of all articles that classified as include or unclear were then obtained, and two authors (MC, GD) examined compliance of reviews with eligibility criteria, with a third author acting as an arbiter (RF).
Data extraction
Data from reports of all included systematic reviews were extracted by two authors (MC, GD) and validated by a third author (RF), using a data extraction form designed and pre-piloted for this overview. The following general characteristics were extracted from each systematic review: publication details; study objectives; eligibility criteria (population, intervention, comparators, outcomes, study designs); any reported protocol; and methods used for search, screening, data extraction and synthesis. For each systematic review, we listed all included studies and evaluated whether they matched the eligibility criteria for this overview regarding population, interventions and outcomes. We then extracted the following results from the eligible group of studies, whenever reported at the systematic review level: study design; countries involved; characteristics of included participants (demographics, prevalence of HIV/HCV); description of interventions and any co-intervention, duration of intervention and follow-up; effect estimates from meta-analysis (if available) or at individual study-level, for each relevant outcome; and any subgroup or sensitivity analyses. The authors’ conclusions for each relevant outcome were also collected. We contacted the authors of reviews for relevant missing data.

Assessment of methodological quality
At a study level, we extracted data on any risk of bias assessments of primary studies when performed and reported by reviewers in each systematic review, including tools used and summarized results. We also collected data on any reported evaluations of the quality of evidence concerning our outcomes of interest in included reviews, particularly those using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool [ 18 ], as well as data on assessments of publication bias.
At a systematic review level, we assessed the methodological quality of each included systematic review using the Risk Of Bias In Systematic Reviews (ROBIS) tool [ 19 ]. Two review authors (MC, RF) performed quality assessments independently, using piloted decision rules. Disagreements regarding overall assessments were resolved through discussion, with a third reviewer serving as the final arbitrator (AVC). The rationale behind assessments was documented. We calculated measures of agreement and reliability between raters for each ROBIS domain.
Data synthesis
We stratified the included systematic reviews by: (i) type of outcomes assessed (blood-borne infections, IRB), and (ii) type of analysis (with or without meta-analysis). We summarized data from the included reviews both in text and in summary tables and figures. When meta-analysis was performed, we report pooled estimates using the models and measures of effect reported by systematic review authors, with 95% confidence intervals (95% CI); we did not perform any additional statistical analysis. When reported, the accompanying I 2 values, which describe the percentage of total variation across studies that is due to heterogeneity rather than chance, were collected [ 20 ].
The search and screening process is summarised in Fig. 1 . A total of 667 citations were identified through the various database searches. Three additional records were identified in the reference lists of screened studies. After 37 duplicates were removed, we obtained 633 citations, which were screened by title and abstract. We excluded 582 citations as they did not meet the inclusion criteria, and the remaining 51 were screened full text. Thirty-five citations were further excluded, and two reports [ 21 , 22 ] were unobtainable. Fourteen reports were thus included, corresponding to 13 systematic reviews, as two records referred to the same study [ 12 , 23 ]. Within those publications three were reports from National Institutes/Expert Commitees in the US [ 24 ], UK [ 12 ] and Canada [ 25 ], while 10 were regular papers published in scientific journals.
Screening decisions up to date as of 30Jun2015. Original search date 12May2015
Description of included reviews
Table 1 lists the key characteristics of the 13 included systematic reviews that evaluated the evidence of the effectiveness of NSP for PWID in the community setting. We classified reviews by type of outcomes reported and type of data analysis (Fig. 2 ).
Studies selected for inclusion classified by outcome(s) reported and strategy for data synthesis. *Grey shaded boxes represent the outcome reported in the reviews indicated in the right column. “HIV”, ”HCV” and “Injecting risk behaviours” is used to classify the reviews that reported the impact of NSP in the number of HIV infections, number of HCV infections and change in injection behavior. The reviews are also presented in the right column by strategy used for data synthesis
The number of databases searched per review varied between two and 15, with the three most common sources being MEDLINE, EMBASE and PsycINFO. The dates of last search varied between 1995 [ 26 ] and 2012 [ 27 ]. Of 287 studies included in these 13 reviews, 200 studies matched the eligibility criteria for this overview, corresponding to 133 unique studies (a matrix with included studies by review is available in a supplementary file: Additional file 2 ). We included the complete set of studies from four reviews, as in the remaining reviews at least one study did not fulfill our eligibility criteria [ 27 – 30 ]. The number of relevant studies included per review ranged from three [ 31 , 32 ] to 43 [ 24 ]. While there was some overlap in the included studies between reviews, the majority (68%) was included in one review only.
We found substantial variability in the criteria used by review authors to define and classify study designs, as well as types of designs of included studies. Only four reviews specified the types of study design in their eligibility criteria [ 12 , 26 , 29 , 33 ]. Three reviews included randomized controlled trials (four RCTs overall) [ 12 , 26 , 31 ], while the remaining reviews included cohorts and case-controls (101 overall), as well as other study designs (time series, before/after studies or ecological studies) (95 overall).
Three of the included reviews were restricted to studies conducted in specific countries or economic regions (China, UK, and low- and middle-income countries) [ 28 , 31 , 32 ]. Nine reviews reported data on HIV, eight on HCV, and six on IRB. Seven reviews evaluated more than one of these outcomes. Four reviews used meta-analysis [ 26 , 27 , 32 , 34 ], one of which using individual-participant data [ 32 ]. The remaining nine reviews used narrative synthesis [ 12 , 24 , 25 , 28 – 31 , 33 , 35 ]. The reasons reported by the authors for conducting or not conducting meta-analysis varied between reviews, even when there was a degree of overlap between included studies.
Methodological quality
Seven reviews reported having assessed the methodological quality of included studies [ 12 , 24 , 25 , 27 , 29 , 33 , 34 ]. In two reviews the authors developed ad hoc tools [ 33 , 34 ], while the remaining reviews used previously existing instruments, often with modifications (tools used: Newcastle-Ottawa, GRADE, WHO-Johns Hopkins 9-Point Rigour Scale, Quality assessment tools developed by NICE Centre for Public Health Excellence, England and Wales and the Effective Public Health Practice Project, Canada) [ 12 , 24 , 25 , 27 , 29 ]. Only two instruments were used more than once, and all other instruments varied. Not all reviews reported the results of these assessments; when reported, most studies were considered to have low methodological quality, given methodological weaknesses and biases related to observational study designs. Only one quantitative review [ 27 ] incorporated quality in data synthesis, by conducting a sensitivity analysis. In reviews without meta-analysis, methodological quality and risk of bias was presented descriptively in the interpretation of study results. Two reviews [ 24 , 27 ] reported using the GRADE tool to assess the quality of the body of evidence. One considered the overall quality of evidence as low [ 27 ], while the other, using an adaptation of GRADE, evaluated the quality of evidence as modest to moderate for different outcomes [ 24 ].
Using the ROBIS tool, only two of the included reviews were considered to have low risk of bias, whereas the remaining were considered to have either high ( n = 8) or unclear risk of bias ( n = 3) (summary of results available in a supplementary file: Additional file 3 ). The majority of reviews were rated as having high or unclear risk of bias across all ROBIS domains, i.e. study eligibility criteria, identification and selection of studies, data collection and study appraisal, synthesis and findings. Percentage of agreement between ROBIS raters varied between 77% and 92%, and weighted kappa (quadratic) between 0.82 and 0.95.
The results from the PRISMA-P statement applied to this overview are presented at Additional file 4 .
Effects of NSP
Hiv prevalence and/or incidence.
Of nine reviews that reported data on HIV outcomes, two were at low risk of bias [ 12 , 27 ], and only one performed meta-analysis [ 27 ]. The proportion of studies included in only one of these nine reviews was 71%.
Reviews with meta-analysis
The most recent review by Aspinall et al. included twelve studies (10 cohort, one case-control and one cross-sectional study), and was evaluated as having low risk of bias. The review reported a 34% “risk” reduction of HIV transmission (pooled effect estimate of 0.66; 95% CI: 0.43 to 1.01; n = 10 studies; I 2 = 76%) in individuals exposed to NSP, compared with those who were not, or were less frequently, exposed to NSP. This estimate was obtained by pooling both adjusted and unadjusted effect sizes regardless of study design, in a random effects model, and pooling all types of effect measures (odds ratio, risk ratio, hazard ratio). Sensitivity analyses supported these results, with a statistically significant reduction in HIV transmission associated with NSP exposure in both higher quality studies (0.42; 95% CI: 0.22 to 0.81), and studies reporting relative risks or hazard ratios (0.60; 95% CI: 0.37 to 0.97). Subgroup analyses showed that studies using sequential follow-up (i.e. groups exposed to NSP and groups not exposed followed sequentially, and not concurrently) and studies recruiting post-1990 had lower effect estimates (0.21 [95% CI: 0.11 to 0.41] and 0.52 [95% CI: 0.28 to 0.95], respectively). Further, studies comparing 100% NSP coverage (i.e. clean needle and syringe used for 100% of injections) with <100% NSP coverage generated a pooled effect estimate of 0.58 (95% CI: 0.22 to 1.57). Quality of evidence was considered low using GRADE, and most of these results had substantial heterogeneity.
Reviews without meta-analysis
The quantitative results presented above are supported by most reviews with qualitative summaries of the evidence only, all of which published between 1999 and 2013. Differences between reviews could be found regarding eligibility criteria (e.g. type of NSP, population- vs individual-level outcomes, study design), search and screening methods, quality assessments and interpretation of study results. In most of these reviews, the authors described and contextualized results from each individual study or group of studies regarding HIV outcomes. Some reviews used vote counting to compare the number of positive studies with reduction in HIV transmission, with the number of negative studies. In the following paragraphs, we present the main results of these reviews, starting with those focusing on the impact of different aspects of NSP provision followed by results of reviews focusing exclusively on the comparison between NSP andno NSP.
Regarding aspects of NSP provision, Jones and colleagues reported results from a recent systematic review classified as low risk of bias, that focused on level of coverage, syringe dispensation policies, type of NSP, provision of additional harm-reduction services and of opiate substitution therapy [ 12 ]. Authors judged that the range of study designs, intervention approaches examined and outcomes precluded the use of meta-analysis. Only three studies focused on HIV outcomes. A low-quality study showed no significant trend for HIV prevalence when comparing primary sources of needles (pharmacies, fixed site NSP and van-based NSP), although HIV prevalence was lower among pharmacy users than in participants who reported using van or fixed site NSP (16% vs. 21% and 25%, respectively, p = 0.16) [ 36 ]. Another study [ 37 ] included in this review examined the impact of dispensation policies, and noted a decrease in HIV prevalence (based on testing or self-report) between the period of legal pharmacy syringe purchase and when up to five needles could be exchanged at newly established NSP (35% to 22%; p < 0.05). Finally, one moderate quality cohort study [ 38 ] also included in the review by Jones and colleagues evaluated different levels of harm reduction and found that a full harm reduction strategy (combination of methadone treatment and full participation in NSP) reduced the incidence of HIV when compared to incomplete or no harm reduction (incidence rate ratio: 0.32; 95% CI: 0.17 to 0.62).
In line with these results, a review with an overall unclear risk of bias by Abdul-Quader et al. [ 29 ] identified studies with structural-level NSP, ie interventions in which changes in policy and legal environment have facilitated an increased availability of sterile syringes, and focused on population-level outcomes. The operational definition of structural-level NSP was a minimum 50% coverage of PWID and distribution of 10 or more needles/syringe per PWID per year. Nine studies included in this review reported decreases in HIV prevalence, and three reported decreases in HIV incidence. All studies were non-randomized before-after comparisons or interrupted time series analyses, and most showed evidence of potential selection biases. The authors concluded that these results support NSP as a structural-level intervention to reduce population-level infection.
Four older published reviews with qualitative synthesis and an overall unclear or high risk of bias reported mixed findings regarding the impact of NSP on HIV incidence/prevalence. These reviews provide a historical perspective on the evolution of NSP implementation and evaluation. The first published review by Leonard et al. [ 25 ], was an update of a previous systematic review. Gibson et al. [ 35 ] included six studies published up to 1999 with a range of study designs, participants and settings, reporting on HIV outcomes. The Institute of Medicine’s evidence report [ 24 ], identified 12 relevant studies, including cohort, case-control and ecological designs, as well as studies using mathematical models. Finally, the review by Kall et al. [ 30 ] included 16 studies published up to 2005, only two of which were not included in other reviews from this overview.
All these reviews included landmark prospective cohort studies conducted in Montreal and Vancouver in the 1990s [ 39 , 40 ], which found an association between NSP participation and higher risk of HIV seroconversion. This led Leonard et al. to conclude that there was methodologically weak evidence that NSP were not as effective as previously found in modifying HIV prevalence and incidence among PWID. Kall et al. used vote counting and reported that on most studies assessing seroincidence the effect of NSP was not significant, while four studies investigating seroprevalence at baseline were unfavourable to NSP. The authors also stated that in studies that found positive effects, confounders had not been adequately controlled for. However, based on epidemiological evidence that accumulated progressively, authors of the other reviews highlighted a number of selection biases that could account for these findings, including: the inclusion of high-risk cocaine injectors, who injected more often than heroin users; the limited number of needles and syringes that users could have access to in early NSP; and the ready availability of clean injecting equipment through pharmacies which could have attracted marginalized, particularly high-risk individuals.
Follow-up studies in the same settings, conducted after expanding and adjusting NSP (e.g. by allowing unlimited distribution of needles/syringes, increasing the number of access points, and offering different distribution methods), found no such increase in risk, or a decrease in HIV prevalence. Gibson et al. [ 35 ] used vote counting and considered there was substantial evidence that NSP were effective in preventing HIV seroconversion. Tilson et al. [ 24 ] included four ecological studies that found an association between HIV prevention programmes that include NSP with reduced prevalence of HIV in urban settings. Based on the weakness of these studies designs, this evidence was considered modest using a modified GRADE approach. Further, moderate evidence was found that multi-component HIV prevention programmes that include NSP reduce intermediate HIV risk behaviour.
The Institute of Medicine report highlighted how almost all published studies originate in North America, Western Europe, and Australia [ 24 ]. Two additional reviews were restricted to specific populations by geographical or economical source. Hong and Li summarized evidence from two studies conducted solely in China [ 31 ], while Des Jarlais et al. focused on 13 studies of 11 NSPs with high-coverage conducted in low/middle-income countries [ 28 ]. In both cases, results from included studies generally supported the effectiveness of NSP in reducing HIV. Des Jarlais et al. reported a reduction of HIV prevalence in four studies (from −3% to −15%), of estimated HIV incidence in three studies (from −11/100 to −16/100 person-years at risk), and of newly reported nationwide cases in three national reports (from −30% to −93.3%). Conversely, increases in HIV prevalence were found in two studies (from +5.6% to +15.8%) and one national report (+37.6%) included in the review by Des Jarlais et al. The authors considered that, if high coverage is achieved, NSP appear to be as effective in low/middle-income as in high-income countries [ 28 ].
HCV prevalence and/or incidence
Eight included reviews synthesized the evidence on the use of NSP in preventing HCV prevention in PWID. One was rated as being at low [ 12 ], three at unclear [ 24 , 25 , 29 ] and four at high risk of bias [ 28 , 32 – 34 ], including two reviews that used meta-analysis [ 32 , 34 ]. The proportion of studies included exclusively in one of these reviews was 88%. Both reviews with quantitative and qualitative synthesis showed mixed results.
Two reviews [ 32 , 34 ], both published in 2011 and rated as being at high risk of bias, used meta-analysis.
Hagan et al. [ 34 ] included 7 studies focusing on NSP and HCV outcomes (6 cohort and 1 case-control study), all from North America. The pooled analysis of all studies, using random effects models and with all measures of effect converted to relative risks, showed an increase in the risk of HCV acquisition with NSP (relative risk, 1.62; 95% CI: 1.04 to 2.52). There was considerable heterogeneity (I 2 = 81%), but no subgroup or sensitivity analyses were performed, and study quality was not explicitly reported or considered in the analysis. Authors cautioned against possible volunteer bias in studies which found an association between HCV acquisition and stand-alone NSP participation, given that exchange programmes attract and retain higher-risk PWID.
The second review by Turner et al. performed individual participant-data meta-analysis based on studies solely conducted in the UK [ 32 ]. High NSP coverage (100% versus <100% needles per injection) reduced the risk of new HCV infection (adjusted odds ratio 0.48; 95% CI: 0.25 to 0.93) ( n = 833 participants, from 3 studies). Full harm reduction (on opiate substitution treatment plus high NSP coverage) further reduced the odds of new HCV infection (adjusted odds ratio 0.21; 95% CI: 0.08 to 0.52). Results were adjusted for gender, homelessness, crack injection and duration of injection.
Earlier reviews with qualitative synthesis included many studies which were not included in the previously mentioned reviews with meta-analysis. These reviews reported mixed results mostly summarized using vote counting, and we found distinct interpretations of findings. We first present the main results of reviews that focused on the influence of different aspects of NSP provision, and then results of reviews focusing exclusively on the comparison of NSP versus no NSP intervention.
The low risk of bias review by Jones et al. [ 12 ] focused on the impact of different aspects of NSP provision and included two studies with HCV outcomes. Both of these studies also included HIV outcomes, and results were consistent for both infections: no difference in HCV prevalence was found when comparing primary sources of needles (pharmacies, fixed site NSP and van-based NSP), and a full harm reduction intervention (including NSP with opiate replacement therapy) was found to reduce the incidence of HCV when compared to no harm reduction (incidence rate of 3.5/100 person-years vs. 23.2/100 person-years with an incidence rate ratio of 0.15; 95% CI: 0.06 to 0.40) [ 38 ].
The most recent review by Abdul-Quader et al. [ 29 ] , classified as having an unclear risk of bias, included six structural intervention studies with before-after design and outcome data on HCV biomarkers published up to 2011, all of which reported a reduction in HCV prevalence after NSP implementation. It should be noted, as described above, that these studies were restricted to NSP with high-coverage based on the number of syringes distributed and the number of PWID in the local population, and that only studies with pharmacy sales in conjunction with NSP were included (and not programmes exclusively focused on pharmacy sale or distribution).
While mostly focused on HIV and IRB outcomes, the Institute of Medicine’s report from 2006 [ 24 ] also considered the impact of NSP on HCV prevention. Five studies provided moderate evidence (adjusted GRADE) that NSP had significantly less impact on transmission and acquisition of HCV than on HIV, which was attributed by study authors to the apparent failure of these programmes in providing other injecting equipment. There was however one case-control study that showed a considerable decrease in HCV acquisition. Another of these earlier reviews, by Wright et al. [ 33 ] based on a World Health Organization review [ 41 ], included 12 relevant studies. Authors highlighted how included observational studies from Europe and Australia showed statistically significant reductions in anti-HCV prevalence or incidence in the early 1990s (shortly after the introduction of NSP), but the trend did not continue in the following years. Also, reported negative studies conducted in the US failed to identify an association between NSP and HCV incidence. On the other hand, two studies with expanded harm reduction included in this review, demonstrated a statistically significant reduction in HCV. Authors of this review concluded that a more effective response to HCV prevention in drug users should include provision of new interventions, such as behavioural interventions and distribution of other injecting paraphernalia alongside needle injection material.
The earliest review, by Leonard and colleagues [ 25 ], focused on three studies which, similarly to HIV outcomes, provided conflicting and methodologically weak evidence that NSP were not associated with decreases in the risk of new infections with HCV (one earlier study showing reduced transmission, and two later studies failing to show any association).
Regarding low/middle-income and transitional-economy countries, Jarlais and colleagues [ 28 ] reviewed four relevant studies, and found a decrease in HCV prevalence (range − 4.2% to −10.2%) in three of those studies, while HCV incidence remained stable in the fourth study. The authors considered that, as with HIV, if high coverage was achieved, NSP appeared to be as effective as in high-income countries.
Injecting risk behaviours
Six reviews reported data on IRB outcomes. One was at low risk of bias [ 12 ], two were at unclear [ 24 , 25 ] and three at high risk of bias [ 26 , 32 , 35 ]. Two reviews performed meta-analysis [ 26 , 32 ]. Specific IRB outcomes reported by each review and study varied. The proportion of studies included in only one of these six reviews was 77%.
The systematic review by Turner et al. [ 32 ], considered to be at high risk of bias, included an individual participant-data meta-analysis reporting UK study data on self-reported IRB outcomes of needle sharing and mean number of injections in the last month. Full harm reduction strategy was associated with a reduced risk in both of these outcomes, with an adjusted odds ratio of 0.52 (95% CI: 0.32 to 0.83) for the former, and an adjusted mean difference of −20.8 (95% CI: -27.3 to −14.4) for the latter (both n = 2143 participants).
An earlier review by Cross et al. [ 26 ], classified as having high risk of bias, pooled data from 10 studies (one RCT and nine observational studies) published up to 1995 across a range of IRB outcomes, including sharing and lending syringes, condom use, injecting frequency and bleach use. NSP were effective in reducing high risk drug use and sexual behaviours (weighted group mean 0.28; 95% CI, 0.21 to 0.35) ( n = 1675). The magnitude of the effect size was affected by outcome and study design: the largest group effect of NSP was to reduce sharing, followed by lending and injecting, and results from pre-post-test designs showed larger effects than the randomized study.
The review by Jones et al. provided relevant study results on the possible influence of different aspects of NSP provision on IRB outcomes [ 12 ]. Two poor quality studies included in this review found that higher syringe coverage and participation in opiate substitution therapy alongside NSP reduced injection risk behaviours among drug users. Further, one cohort study and one cross-sectional study suggested that PWID who obtained their needles exclusively from NSP were less likely to engage in high risk behaviours than those who obtained them via secondary distribution, and in turn the latter had less IRB than those who obtained no needles directly or indirectly from NSP. On the other hand, evidence from two RCTs also included in Jones et al. review suggested that NSP setting does not impact on injection risk behaviours. One of these trials compared pharmacy sales only with NSP exchange plus pharmacy sales [ 42 ], and the other compared hospital and community-based NSP [ 43 ]. As supplementary evidence, three poor quality cross-sectional studies found that mobile van sites and vending machines may attract younger PWID with higher risk profiles. Finally, evidence from three cross-sectional studies, also included in this review, suggested that syringe dispensation policies had a limited impact on behavioural outcomes such as sharing, but some impact on syringe re-use.
Reviews with narrative summaries provided largely similar results regarding the overall effect of NSP. Tilson et al. included 18 cohorts in their systematic review that examined the impact of NSP on drug-related risks [ 24 ]. Thirteen studies found a reduction in self-reported needle sharing. One study found injection frequency to decrease, and four other studies showed no difference in this outcome. Authors cautioned that nearly all programmes combined needle and syringe exchange with other components such as outreach, risk reduction education, condom distribution, bleach distribution, education on needle disinfection, and referrals to substance abuse treatment and other health and social services. Evidence was judged to be moderate (adjusted GRADE). The authors reviewed the evidence for pharmacy sales and physician-based prescriptions and concluded that pharmacy dispensation, free of criminal penalty, was an alternative strategy to make sterile needles and syringes available to PWID.
Two earlier reviews [ 25 , 35 ], already described in the previous sections of the results, also analysed IRB outcomes. Gibson et al. identified 23 studies published up to 1999, and using vote counting concluded that there was substantial evidence that NSP are effective in preventing IRB. Leonard et al. reviewed 19 low quality primary studies and evidence supported an effect of NSP in modifying risk related injection practices.
Summary of main results
In this overview of systematic reviews examining the effectiveness of NSP for PWID in reducing blood-borne infection transmission and injecting risk behaviours, we identified 13 systematic reviews contributing with 133 unique studies, which were mostly observational. Methods used in these reviews varied at all levels of review design and conduct. Only two reviews were considered to have low risk of bias by reviewers [ 12 , 27 ] and most included studies were evaluated as having low methodological quality. The quality of evidence, when assessed, was considered low or modest to moderate. Nine reviews reported outcome data on HIV prevalence/incidence, eight on HCV, and six on IRB. Meta-analysis was performed in four of these reviews.
Our interpretation of the findings is that the overall results of the included systematic reviews are supportive of the effectiveness of NSP in reducing HIV transmission and IRB among PWID, as well as in reducing HCV infection, although the latter to lesser extent. The overall quality of the evidence is higher for HIV transmission and IRB than for HCV infection. However, for HCV infection, the strength of the evidence increases (because studies’ results are more consistent) if the intervention under consideration is not solely NSP, but includes other components such as opiate replacement treatment, in a strategy of full harm reduction intervention.
Furthermore it is well known that sharing other injecting equipment (e.g. swabs, cookers, water and filters) is an important route of transmission of blood-borne infections, particularly in the case of HCV. In addition, clean injecting equipment is also available through sources other than NSP (dilution bias) [ 44 , 45 ]. This overview did not identify any studies evaluating the effects of paraphernalia distribution at reducing the incidence or prevalence of HCV. One further aspect is that individual NSP intervention studies are prone to selection (volunteer) bias, as these exchange programmes attract and retain higher-risk PWID. Taken together, these aspects may have contributed to some mixed results reported in the systematic reviews and individual studies addressing HCV infection.
To sum up, aspects of NSP provision may be relevant, including structural-level NSP (i.e. high-level coverage), and multi-component programmes including full harm reduction seem to benefit all outcomes more than individual NSP.
Overall completeness and applicability of evidence
In this field of public health, interventions and results are highly influenced by economic, legal, ethical, social and cultural circumstances. In the past, results from some of the included studies led to major political decisions and fuelled controversial debates, particularly in the US. There are considerable differences in perspectives regarding the role and provision of harm reduction services, which impact study design and conduct and selection of participants, and thus affect the validity and generalizability of study results. This may at least partially explain why we found heterogeneity in study results and time trends, although there was consistency in many outcomes. Further, while the majority of the published research presented in different reviews originated from North America and Europe, one of the reviews [ 28 ] supported part of these results for both high and low/middle-income countries. Importantly, provision of NSP interventions is low at a global level, particularly in countries where the incidence and prevalence of both HIV and HCV is highest.
Aspects of NSP provision are also key to decision-making, and few included reviews examined how different types of NSP provision impact on effectiveness. NSP are extremely diverse in their design, staffing, characteristics of participants, operation and programme delivery policies. Further, PWID are diverse populations with different preferences, behaviours, and life circumstances, who often have difficulty in accessing formal healthcare services. As such, the potential impact of NSP in reducing injecting related-harms is limited by the extent to which the programmes provide effective access to sterile injecting equipment and other services, and are therefore able to attract their potential clients [ 46 , 47 ].
Results from this overview support the importance of high-level coverage and comprehensive services provided by many NSP in reducing bloodborne infections and risk behaviours. We should note that outcomes which were not included in this review may be relevant to evaluate possible benefits of NSP. For example, pharmacy access to sterile needles and syringes has been found to provide specific benefits in addition to those available through specialist NSPs [ 12 ]. Importantly, the coexistence of different modes of injecting equipment delivery, as well as tailoring services offered at different venues, addresses several barriers encountered by PWID, as modalities for improving syringe availability are likely complementary and not competitive [ 48 – 50 ].
The additional benefits provided through this complementary role of pharmacies may have prompted Governments of Australia, Ireland, Spain (Basque Country) and UK to remunerate pharmacies for the important public health role provided through NSP [ 8 , 51 – 55 ]. As pharmacy-based NSP gradually expand, it is important to pursue future research with a focus on this setting.
Quality of the evidence
The results hereby presented have to be treated with considerable caution. The methodological quality of the included systematic reviews varied, but a majority of reviews were considered to be at high risk of bias. Importantly, we found considerable heterogeneity and inadequacies in methods used by different reviews, at all stages of the design, conduct and reporting of the review. Differences between eligibility criteria and search and screening methods likely explain a striking mismatch between included studies, despite some reviews having similar research questions and covering relatively close publication periods. Assessment of quality of included studies was seldom performed, with the use of different tools for assessment of risk of bias, some of which were developed ad hoc with no validation, and hardly any use of grading of quality of evidence. We also found variability in the decision as to whether or not to perform quantitative synthesis, even when the body of evidence was similar. Few review authors chose to present pooled estimates from meta-analyses, and when present, there was variation in models, choice of outcome measures and use of sensitivity and subgroup analyses. Other authors opted to present narrative summaries of results, often with vote counting, but there were discrepancies in the interpretation of trial results. Thus, there were obstacles in summarizing, comparing and synthesizing results from all included reviews.
These factors also reflect the challenges that review authors had in summarizing results from individual studies, most of which were judged to be of low methodological quality. Classification of study design was discrepant between reviews, but most studies were observational, at either individual or population-level. These designs are limited in establishing causality, and we found different approaches regarding adjustment for possible mediators and confounders. Further, a range of selection biases were identified and explored in some landmark studies, including volunteer bias of highest risk users into NSP. Less biased evaluations of NSP would require the use of randomization, for example at a cluster level, but there are many ethical, scientific and practical challenges. Other aspects to consider are the challenges in selecting, comparing and pooling different outcome measures and measurement tools when assessing HIV and HCV infection, as well as needle and syringe sharing and injecting frequency. A final limitation of this body of evidence is that studies generally were not designed to allow for separate examination of different aspects of NSP provision. As most public health interventions, NSP are complex health interventions with several interacting components, multilevel factors (users, providers, setting, health systems), and some degree of flexibility of interventions allowed in the real-world setting. Hence, challenges will likely persist in identifying the independent contribution of improving access to sterile needles and syringes, both at review and individual study level.
Agreements and disagreements with other reviews and potential biases
Two other overviews of reviews were published on this topic [ 12 , 14 ]. The first overview, published in 2008 [ 12 ], was undertaken as a section of an extensive review of the effectiveness and cost-effectiveness of NSPs for PWID to inform guidance on the optimal provision of NSPs from the perspective of the UK National Health Service. The authors concluded that none of the included systematic reviews examined HCV in any depth and that there was insufficient evidence to support the effectiveness of NPS on HCV incidence/prevalence. On the other hand, there was tentative and sufficient evidence to support the effectiveness of NSP on HIV incidence/prevalence and on self-reported IRB, respectively. The second overview was an updated overview published in 2014 [ 13 , 14 ] and focused on the effectiveness of different harm reduction interventions, including NSP, in preventing IRB, HIV and HCV transmission among PWID [ 14 ]. This review was related to guidance from the European Centre for Disease Prevention and Control and the European Monitoring Centre for Drugs and Drug Addiction. Taking into consideration the individual conclusions of the core included systematic reviews, as well as the number of individual studies showing positive findings (i.e. a reduction of IRB, HIV and/or HCV incidence/prevalence), the authors concluded that there was: 1) sufficient review-level evidence of effectiveness in relation to IRB; 2) tentative review-level evidence to support the effectiveness of NSP in reducing HIV transmission among PWID; and 3) insufficient review-level evidence to support the effectiveness of NSP in reducing HCV transmission.
The field of overviews is relatively new, and this type of study design has been gaining momentum as a valuable knowledge synthesis methodology that can collate extensive information to facilitate the uptake and application of knowledge by decision-makers [ 56 ]. However, published overviews show considerable variation in their methods and reporting due to the unique methodological challenges inherent in summarizing and synthesizing evidence from different heterogeneous sources [ 57 , 58 ]. Reviews in public health pose additional challenges, as methods are likely to vary more, with less quantitative analysis and different approaches to synthesizing and interpreting evidence.
Previous overviews have used different approaches to review eligibility, selection, quality assessment and analysis. Also, there were differences in the framework used to classify included reviews (e.g. core/supplementary), the type of summary and synthesis used (e.g. vote counting according to study or review results), and the conclusions and evidence statements. In comparison, the strengths of our overview include the use of strict eligibility criteria, the evaluation of methodological quality of included systematic reviews, and the categorization of review by type of analysis and outcome. We used the novel ROBIS tool [ 19 ], which was developed and validated to overcome limitations seen in previous instruments to assess the conduct and reporting of systematic reviews. This tool allowed us to stratify review quality using clear and transparent criteria, thus providing support to our confidence in each review’s findings. Further, by presenting pooled estimates of the effect of NSP in different outcomes taken from reviews that performed meta-analysis, we provide the opportunity for readers to quantify the direction and magnitude of this effect. We also highlight the need to use this quantitative data to assess the likely relevance of findings.
There are a number of limitations to our overview. While we identified and listed all unique studies included in each review and assessed the degree of overlap between reviews, we did not assess these studies individually, as other overviews have [ 57 ]. We focused on results from different reviews, which had some degree of overlap, and thus have a risk of double counting results, both for qualitative and quantitative reviews. Further, the application of ROBIS was challenging, although measures of agreement between raters were relatively adequate, likely due to the use of piloted decision rules.
The findings of this overview of systematic reviews allow the conclusion that there is moderate quality evidence that NSP is likely effective in reducing HIV transmission and IRB among PWID, and that there is low to moderate quality evidence that NSP in the context of a comprehensive harm reduction strategy is likely effective in reducing HCV infection. Full harm reduction interventions provided at structural level and in multi-component programmes seem to be more beneficial. The scarcity and the lack of robust quality of evidence highlights the need for future community-level studies of adequate design to support these conclusions, as well as to address the impact of different aspects of NSP provision. Future reviews and possibly overviews of reviews should use standardized methods and frameworks to improve comparability, synthesis and interpretation of findings.
Abbreviations
Case-Control study
Cochrane Database of Systematic Reviews
Centre for Health Evaluation & Research
Cohort study
Database of Abstracts of Reviews of Effects
Campbell Library of Systematic Reviews and the Database of Promoting Health Effectiveness Reviews
Grading of Recommendations Assessment, Development and Evaluation
Hepatitis B virus
Hepatitis C virus
Human Immunodeficiency Virus
injecting risk behaviours
National Health System Economic Evaluation Database
National Institute for Health and Clinical Excellence
- Needle and syringe programmes
other study design (time-series designs, before-after studies, ecological studies)
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
people who inject drugs
Randomized Control Trial
UNAIDS: UNAIDS: the gap report. 2014.
Google Scholar
WHO. Guidance on prevention of viral hepatitis B and c among people who inject drugs. Geneva: WHO; 2007.
Torre C. Syringe exchange Programmes in the context of harm reduction. Arq Med. 2009;23:119–31.
Ball AL, Rana S, Dehne KL. HIV prevention among injecting drug users: responses in developing and transitional countries. Public Health Rep. 1998;113 Suppl:170–81.
van den Hoek JA, van Haastrecht HJ, Coutinho RA. Risk reduction among intravenous drug users in Amsterdam under the influence of AIDS. Am J Public Health. 1989;79:1355–7.
Article PubMed PubMed Central Google Scholar
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: new guidance. London: Medical Research Council; 2008.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European drug report: data and statistics. Lisbon: European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); 2015.
The Pharmacy Guild of Australia: Community pharmacy roadmap program development template. Needle and syringe program. 2010; Barton: The Pharmacy Guild of Australia.
Gay Men’s Health Crisis. Syringe exchange programs around the world: global context. New York: Gay Men’s Health Crisis; 2009.
National Institute for Health and Care Excellence (NICE). Needle and syringe Programmes, NICE public health guidance, vol. 52. London: National Institute for Health and Care Excellence; 2014.
Sheridan J, Henderson C, Greenhill N, Smith A. Pharmacy-based needle exchange in New Zealand: a review of services. Harm Reduct J. 2005;2:1–9.
Article Google Scholar
Jones L, Pickering L, Sumnall H, Mcveigh J, Mark A, Bellis M. A review of the effectiveness and cost-effectiveness of needle and syringe Programmes for injecting drug users. 2008, Liverpool: Centre for Public Health, Liverpool John Moores University.
Palmateer N, Kimber J, Hickman M, Hutchinson S, Rhodes T, Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction. 2010;105:844–59.
Article PubMed Google Scholar
MacArthur GJ, van Velzen E, Palmateer N, Kimber J, Pharris A, Hope V, Taylor A, Roy K, Aspinall E, Goldberg D, Rhodes T, Hedrich D, Salminen M, Hickman M, Hutchinson SJ. Interventions to prevent HIV and hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25:34–52.
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org/ .
Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York; 2008.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Takacs I, Demetrovics Z. The efficacy of needle exchange programs in the prevention of HIV and hepatitis infection among injecting drug users. Psychiatr Hung. 2009;24:264–81.
PubMed Google Scholar
De Lima M, Justo L, Formigoni M. Needle exchange in Sao Paulo city: a harm reduction strategy for injection drug users. J Bras Psiquiatr. 2005;54:286–92.
Jones L, Pickering L, Sumnall H, McVeigh J, Bellis MA. Optimal provision of needle and syringe programmes for injecting drug users: a systematic review. Int J Drug Policy. 2010;21:335–42.
Tilson H, Aramrattana A, Bozzette S, Celentano D, Falco M, Hammett T. Preventing HIV Infecrtion among injecting drug users in high risk countries: an assessment of the evidence. Washington: Institute of Medicine of the National Academies; 2006.
Leonard L, Forrester L, Navarro C, Hansen J, Doucet C. The effectiveness of needle exchange programs in modifying HIV-related outcomes: a systematic review of the evidence 1997–1999. Hamilton: Effective Public Health Practice Project (EPHPP); 1999.
Cross J, Saunders CM, Bartelli D. The effectiveness of educational and needle exchange programs: a meta-analysis of HIV prevention strategies for injecting drug users. Qual Quant. 1998;32:165–80.
Aspinall E, Nambiar D, Goldberg D, Hickman M, Weir A, Van Velzen E, et al. Are needle and syringe programmes associated with a reduction in hiv transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:235–48.
Des Jarlais DC, Feelemyer JP, Modi SN, Abdul-Quader A, Hagan H. High coverage needle/syringe programs for people who inject drugs in low and middle income countries: a systematic review. BMC Public Health. 2013;13:53.
Abdul-Quader AS, Feelemyer J, Modi S, Stein ES, Briceno A, Semaan S, Horvath T, et al. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav. 2013;17:2878–92.
Kall K, Hermansson U, Amundsen EJ, Ronnback K, Ronnberg S. The Effectiveness of Needle Exchange Programmes for HIV Prevention A Critical Review. J Glob Drug Policy Pract. 2007;1(3). http://www.globaldrugpolicy.org/Issues/Vol%201%20Issue%203/The%20Effectiveness%20of%20Needle%20Exchange.pdf . Accessed 20 Aug 2015.
Hong Y, Li X. HIV/AIDS behavioral interventions in China: a literature review and recommendation for future research. AIDS Behav. 2009;13:603–13.
Turner KME, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978–88.
Wright NMJ, Tompkins CNE. A review of the evidence for the effectiveness of primary prevention interventions for hepatitis C among injecting drug users. Harm Reduct J. 2006;3:27.
Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204:74–83.
Gibson DR, Flynn NM, Perales D. Effectiveness of syringe exchange programs in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS. 2001;15:1329–41.
Article CAS PubMed Google Scholar
Miller CL, Tyndall M, Spittal P, Li K, Palepu A, Schechter MT. Risk-taking behaviors among injecting drug users who obtain syringes from pharmacies, fixed sites, and mobile van needle exchanges. J Urban Health. 2002;79:257–65.
Singer M, Himmelgreen D, Weeks MR, Radda KE, Martinez R. Changing the environment of AIDS risk: findings on syringe exchange and pharmacy sales of syringes in Hartford, CTs. Med Anthropol. 1997;18:107–30.
Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam cohort studies among drug users. Addiction. 2007;102:1454–62.
Bruneau J, Lamothe F, Franco E, Lachance N, Désy M, Soto J, et al. High rates of HIV infection among injection drug users participating in needle exchange programs in Montreal: results of a cohort study. Am J Epidemiol. 1997;146:994–1002.
Strathdee S, Patrick D, Currie S, Cornelisse P, Rekart M, Montaner J, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11:f59–65.
Wright N, Millson C. What is the evidence for the effectiveness of interventions to reduce hepatitis C infection and the associated morbidity. Copenhagen: World Health Organization Europe; 2005.
Fisher DG, Fenaughty AM, Cagle HH, Wells RS. Needle exchange and injection drug use frequency: a randomized clinical trial. J Acquir Immune Defic Syndr. 2003;33:199–205.
Masson C, Sorensen J, Perlman D, Shopshire M, Delucchi K, Chen T, et al. Hospital- versus community-based syringe exchange: a randomized controlled trial. AIDS Educ Prev. 2007;19:97–100.
Cox J, De P, Morissette C, Tremblay C, Stephenson R, Allard R, et al. Low perceived benefits and self-efficacy are associated with hepatitis C virus (HCV) infection-related risk among injection drug users. Soc Sci Med. 2008;66:211–20.
Heinzerling KG, Kral AH, Flynn NM, Anderson RL, Scott A, Gilbert ML, et al. Human immunodeficiency virus and hepatitis C virus testing services at syringe exchange programs: availability and outcomes. J Subst Abus Treat. 2007;32:423–9.
Kral AH. What is it about needle and syringe programmes that make them effective for preventing HIV transmission? Int J Drug Policy. 2003;14:361–3.
Small W. Examining barriers to syringe access among injection drug users. Int J Drug Policy. 2005;16:291–2.
WHO. Policy guidelines for collaborative TB and HIV Services for Injecting and Other Drug Users – an integrated approach. Geneva: World Health Organization, United Nations Office on Drugs and Crime; 2008.
Coffin P. Syringe availability as HIV prevention: a review of modalities. J Urban Health. 2000;77:306–30.
Article CAS PubMed PubMed Central Google Scholar
Moatti JP, Vlahov D, Feroni I, Perrin V, Obadia Y. Multiple access to sterile syringes for injection drug users: vending machines, needle exchange programs and legal pharmacy sales in Marseille, France. Eur Addict Res. 2001;7:40–5.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Harm reduction overview for Ireland. Lisbon: European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); 2016.
Programa de Intercambio de Jeringuillas, País Vasco. http://www.observatoriocarteraservicios.com/iniciativas/programa-de-intercambio-de-jeringuillas-pais-vasco# . Accessed 29 Dec 2015.
NHS England: Improving Health and Patient Care through Community Pharmacy - Evidence Resource Pack. 2013; London. https://www.england.nhs.uk/wp-content/uploads/2013/12/comm-pharm-res-pack.pdf . Accessed 29 Dec 2015.
Community Pharmacy Contract - Enhanced Services. http://www.wales.nhs.uk/sites3/page.cfm?orgid=498&pid=7552 . Accessed 29 Dec 2015.
NHS Care Services. Local Services. http://www.communitypharmacyscotland.org.uk/nhs-care-services/services/local-services/ . Accessed 29 Dec 2015.
Hartling L, Vandermeer B, Fernandes RM. Systematic reviews, overviews of reviews and comparative effectiveness reviews: a discussion of approaches to knowledge synthesis. Evid-Based Child Heal. 2014;9:486–94.
Hartling L, Chisholm A, Thomson D, Dryden DM. A descriptive analysis of overviews of reviews published between 2000 and 2011. PLoS One. 2012;7:e49667. http://dx.doi.org/10.1371/journal.pone.0049667 .
Pieper D, Buechter R, Jerinic P, Eikermann M. Overviews of reviews often have limited rigor: a systematic review. J Clin Epidemiol. 2012;65:1267–73.
Download references
Acknowledgements
Not applicable.
This study was funded by the Portuguese National Association of Pharmacies (ANF). ANF had no control on the conduct or publication of this research.
Availability of data and materials
All relevant data are available in the paper and its supporting information files.
Authors’ contributions
RF and AVC were the guarantors. All authors contributed to the study protocol, the development of the selection criteria, the risk of bias assessment strategy, and data extraction criteria. JA and MC developed the search strategy. CT and SC provided content expertise on NSP. MC and GD examined compliance of reviews with eligibility criteria, with a third author acting as an arbiter (AVC or RF). MC and GD extracted data from reports of all included systematic reviews which was validated by a third author (RF). GJ contributed to the study inclusion, data extraction, and result interpretation and discussion. MC and RF performed quality assessments independently with a third reviewer serving as the final arbitrator (AVC). JC contributed to the interpretation and discussion of results. All authors read, provided feedback and approved the final manuscript. All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
CT, MC and SC are employed by the Portuguese National Association of Pharmacies (ANF). GJ, JA, JC and AVC, affiliated to the Center for Evidence-Based Medicine, and CT, MC and SC, affiliated to the Centre for Health Evaluation & Research are involved in the economic evaluation of Needle and Syringe Exchange Programme in Portugal. RF and GD declare that they have no competing interests.
Consent for publication
Ethics approval and consent to participate, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Center for Evidence-Based Medicine, Faculty of Medicine, University of Lisbonl, Av. Prof. Egas Moniz, 1649-028, Lisbon, Portugal
Ricardo M Fernandes, Gonçalo Duarte, Gonçalo Jesus, Joana Alarcão, João Costa & António Vaz Carneiro
Centre for Health Evaluation & Research (CEFAR), National Association of Pharmacies, Rua Marechal Saldanha, n°1, 1249-069, Lisbon, Portugal
Maria Cary, Carla Torre & Suzete Costa
Portuguese Collaborating Centre of the IberoAmerican Cochrane Network-Cochrane Portugal Faculty of Medicine, University of Lisbon, Av. Prof. Egas Moniz, 1649-028, Lisbon, Portugal
Ricardo M Fernandes, João Costa & António Vaz Carneiro
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to António Vaz Carneiro .
Additional files
Additional file 1:.
Search Strategies. (DOCX 19 kb)
Additional file 2:
List of primary studies included in each review. (DOCX 26 kb)
Additional file 3:
Summary results from ROBIS evaluation performed by two assessors (MC&RF). (DOCX 13 kb)
Additional file 4:
Prisma Checklist. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Fernandes, R.M., Cary, M., Duarte, G. et al. Effectiveness of needle and syringe Programmes in people who inject drugs – An overview of systematic reviews. BMC Public Health 17 , 309 (2017). https://doi.org/10.1186/s12889-017-4210-2
Download citation
Received : 01 July 2016
Accepted : 31 March 2017
Published : 11 April 2017
DOI : https://doi.org/10.1186/s12889-017-4210-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Harm reduction interventions
- People who inject drugs
- HIV/Aids-Hcv-Hbv
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]

IMAGES