
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

14A: Atomic Emission Spectra (Experiment)
- Last updated
- Save as PDF
- Page ID 95880

- Santa Monica College
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
- Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light.
- Measure several wavelengths of light emitted by a polyelectronic element and compare the measured values to actual values.
- Measure the wavelengths of light emitted by hydrogen and identify the electronic transition that gave rise to each wavelength using Bohr’s theory.
Atomic Emission Spectra
Electrons in atoms normally occupy the lowest energy states possible. Such an atom is said to be in the ground state. However, electrons can be excited to high energy states when they absorb excess energy. The excess energy can be provided by heat, light, or electrical discharge. The electrons then return to lower energy states, eventually returning all the way to the ground state. As the electrons return to lower energy states, they release their excess energy. Often, this excess energy is released in the form of light, with each atom or molecule releasing a single photon of light for each electron energy transition it makes.
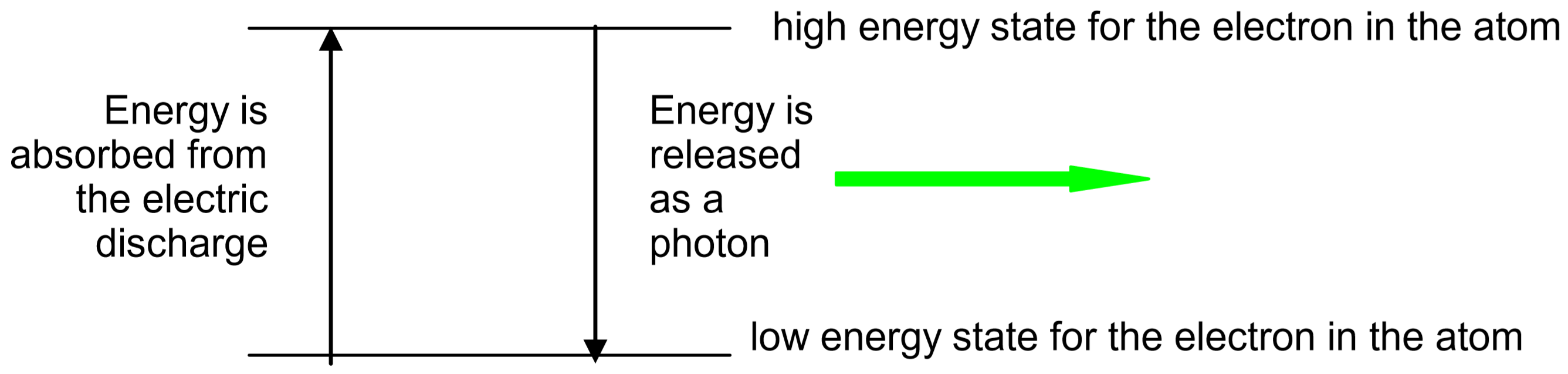
In the hydrogen discharge tubes used in this experiment, the energy of the electric discharge first dissociates the \(\ce{H2}\) molecules into \(\ce{H}\) atoms, then excites the electrons in the \(\ce{H}\) atoms into high energy states.
Due to conservation of energy, the amount of energy in an emitted photon will exactly match the amount of energy lost by the electron as it moves to the lower energy state. Different colors of light are associated with different photon energies. For example, a single photon of blue light has more energy than a single photon of red light. Thus, the color of the light emitted by a particular atom depends upon how much energy the electron releases as it moves down to a lower energy level. The energy levels that are allowed for each atom depend upon the number and arrangement of protons and electrons in the atom. Thus, each element has different energy states available to it, so each element releases photons of different color when its atoms return to their lower energy states. Since each atom has many excited states (high energy levels) available to it, several colors of light can be emitted by each element. The set of individual colors emitted by an element is called its spectrum. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify unknown elements.
Wavelengths of Light
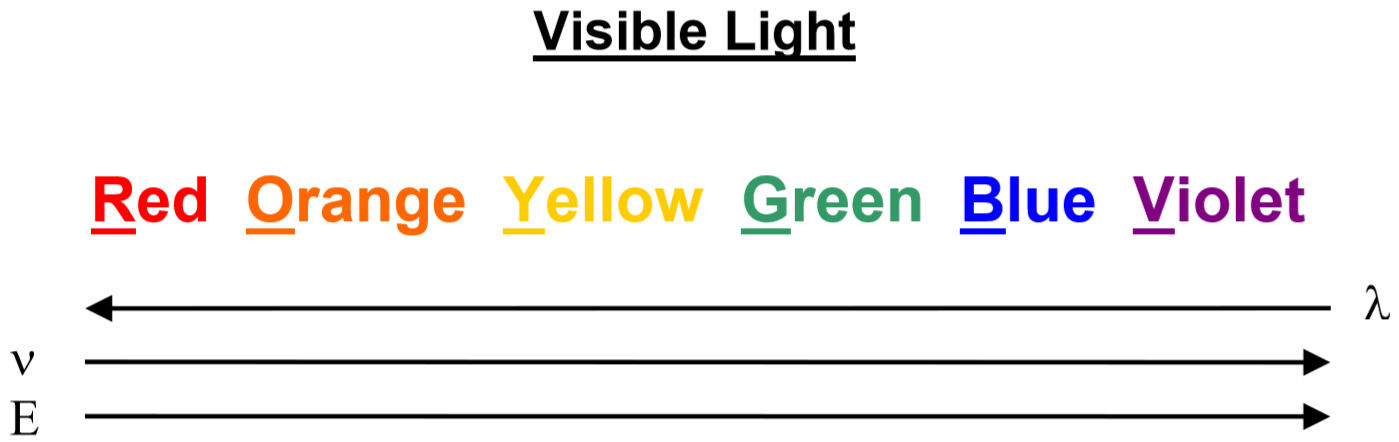
Light is one kind of electromagnetic radiation. The wavelength of radiation determines what kind of radiation it is. The human eye is able to detect only a narrow range of wavelengths of electromagnetic radiation, those from about 400nm to about 700nm. Radiation with wavelengths less than 400nm is classified as ultraviolet, x-ray, or \(\gamma\)-rays, while radiation with wavelengths longer than 700 nm is classified as infrared radiation, microwaves, and radio waves. In this experiment, we use our eyes to detect the radiation emitted by excited atoms and, therefore, we work only with visible light
The color of light is related to its wavelength (\(\lambda\)), which is related to its frequency (\(\nu\)) and the energy of its photons (\(E\)). Shorter wavelengths of light (at the blue end of the visible spectrum) have higher frequencies and higher photon energies while longer wavelengths of light (at the red end of the spectrum) have lower frequencies and less energy per photon.
It is easy to convert between photon energy, wavelength, and frequency using the following relationships where \(c\) = the speed of light = \(2.998 \times 10^8 m/s\) and \(h\) = Planck’s Constant = \(6.626 \times 10^{-34} Js\).
\[\lambda \nu = c\]
\[E = h \nu\]
These two relationships combine to give a third:
\[E = \dfrac{hc }{\lambda}\]
Thus, the spectrum of an element can be stated by listing the particular wavelengths of light that its atoms emit.
To measure these wavelengths in the laboratory, we must first separate them. To the naked eye, the various wavelengths (colors) of light emitted by an element are mixed together and appear as a single color that is a combination of the component colors. If we view the light through a prism or a diffraction grating, however, the individual wavelengths are separated. A diffraction grating is a piece of glass or clear plastic with many very narrow and closely spaced lines on it. As the light emerges after being reflected by the grating, these tiny lines cause the reflected light to interfere with itself in such a way that the different wavelengths of the light to appear in different positions to the left and right of the original direction in which the light was traveling. See the figure below.
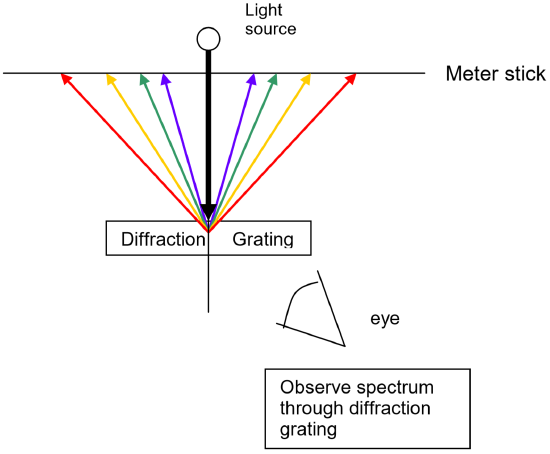
Using a light source that contains known wavelengths of light, we can measure exactly where each known wavelength appears along a meter stick. Since this position depends upon the wavelength in a linear way, a graph of wavelength vs. position of the spectral line will yield a straight line. Once the best fit straight line has been determined, the equation of this line can then be used to convert positions of other spectral lines to wavelength. For example, using the same apparatus and without moving the relative positions of the meter sticks, diffraction grating and lamp, it is possible to view the spectrum of a new element, measure where its spectral lines occur on the meter stick, and then read the graph or use the equation of the line to determine the wavelength to which each of those positions corresponds. The calibration graph is therefore an integral part of the spectroscope. Positions are measured using the meter sticks, then wavelengths are determined from the positions using the graph itself or the equation of the best fit line for that graph.
Bohr’s Theory
For atoms that contain only one electron, the theory of atomic structure proposed by Niels Bohr can be used to calculate wavelengths for transitions between particular electronic energy levels of the atom. In this experiment, the only one-electron atom we will consider is hydrogen. (Note, there are other one-electron “atoms” if you consider ions such as \(\ce{He^+}\), \(\ce{Li^{2+}}\), etc.)
Using Bohr’s theories for hydrogen, you should find a close match between the calculated wavelengths and those that you measure experimentally. To calculate the wavelengths of light emitted by hydrogen atoms, recall that the energy of an electron in the n-th energy level of a one-electron atom is given by:
\[E_n= -\dfrac{Z^2R}{n^2}\]
where \(R\) is the Rydberg constant = \(2.18 \times 10^{-18} J\), \(Z\) is the nuclear charge, and n = 1, 2, 3, ..., ∞. For hydrogen, the nuclear charge is 1 so this equation becomes:
\[E_n= -\frac{R}{ n^2}\]
The change in energy for the electron when it makes a transition from one level to another is given by its subtracting its initial energy from its final energy:
\[\Delta E_{\text{electron}} = E_f - E_i\]
By conservation of energy, the energy of the photon emitted as this electron drops to a lower energy level must equal the change in energy for the electron. However, since photon energies must be a positive quantity, the absolute value of the change in energy for the electron must be used:
\[E_{\text{photon}} = | \Delta E_{\text{electron}} |\]
Once the energy of the photon is known, it is readily converted into a wavelength as discussed earlier:
\[E_{\text{photon}} = \frac{hc}{\lambda}\]
or
\[ \lambda = \frac{hc}{E_{\text{photon}}}\]
Because there are many energy levels possible for the electron in a hydrogen atom, and because the electron could jump from any higher n to any lower n, there are many lines in the spectrum of hydrogen. However, most of these lines occur at wavelengths which our eyes cannot detect (either infrared or ultraviolet). The visible portion of the spectrum which you will observe in this experiment was the first to be studied by scientists since it is the only portion which can be seen with the naked eye. This series of spectral lines is named for one of the first scientists to study it and is called the Balmer series. Note that all of the spectral lines in the Balmer series involve transitions from a higher n level to the n=2 level. You will need that information to complete the calculations for your lab report.
Materials and Equipment
High voltage power supply; hydrogen, mercury, and other polyelectronic element discharge tubes; meter sticks bolted in a T shape; a diffraction grating; a flashlight, ring stands.
- Use extreme caution near the high voltage power supply! Severe shocks are possible. Do not touch the front of the power supply while it is plugged in! Be sure to turn it off AND unplug it before changing discharge tubes.
- Allow discharge tubes to cool before attempting to remove them from the power supply. They become very hot with use.
- View the light emitted by the discharge tubes through glasses or goggles. Both glass and plastic lenses will absorb most of the harmful UV rays emitted by many atoms.
Part A: Calibration of the Spectroscope Using Known Wavelengths
Work in groups of 4 unless instructed otherwise. Select a workspace on one of the bench tops away from other light sources.
- Obtain 3 ring stands and adjust the iron rings so they are all at exactly the same height, about 6 inches above the bench top.
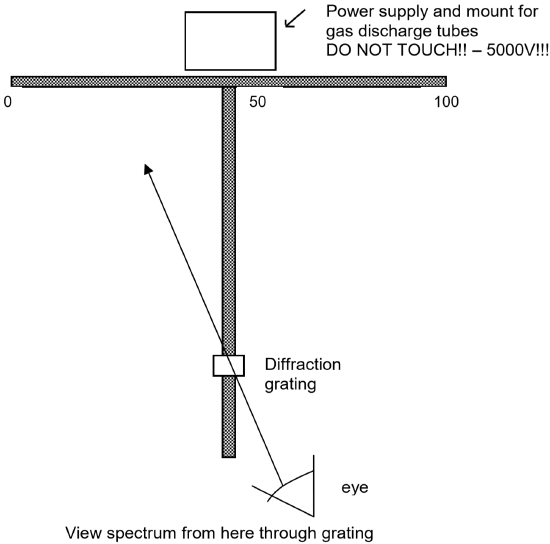
- Obtain a pair of meter sticks that have been bolted together in a “T” shape. Place the ring stands under the ends of the meter sticks so the meter stick arrangement is held about 6 inches above the bench top an is level.
- Place a high voltage power supply ( 5000 V - DANGER!! - DO NOT TOUCH IF PLUGGED IN!! ) containing a mercury discharge tube at the intersection point of the two meter sticks as shown in the figure on the next page. (Note that this is a very high voltage power supply! You must be careful never to touch it when it is plugged in. When you need to insert or remove a discharge tube, turn it off AND unplug it before touching the tube. Also note that the tubes become hot from use. You must let them cool before attempting to remove them.)
- Mount a diffraction grating held by a rubber stopper in a utility clamp attached to a ring stand. Place the ring stand so the diffraction grating is centered over the vertical meter stick and located about 20 cm from the free end of the vertical meter stick. See the following figure illustrating the experimental apparatus.
- Be sure not to bump the meter sticks, ring stands, or diffraction grating! If any of these components is moved in the experiment, the results will be less accurate. You will need to move the power supply to change discharge tubes, so it is a good idea to mark it’s initial position with masking tape so you can be sure to put in back in the same position each time.
- Be sure you have a mercury (\(\ce{Hg}\)) discharge tube in your power supply then turn it on. The spectrum of mercury is well known. It contains four visible wavelengths that are easily seen:
When you look at the mercury lamp through your diffraction grating, you should see each of these four colors at varying positions along your horizontal meter stick. You can use either the spectrum to the left of the lamp or to the right. It doesn't matter because they are the same, but you should be consistent for the rest of the experiment.
- Measure the distance in cm of each line in the mercury spectrum from the center of the meter stick where the lamp is located. Note that the center of the meter stick is at 50 cm so you will have to compensate for that. Record these positions in Table 1 on your data sheet.
- Use Excel to make a graph of wavelength (in nm) vs. position (in cm) from your four data points for mercury. Wavelength should be on the y axis, position on the x axis. Obtain the equation of the best fit straight line for these data and record it on your data sheet. For help using Excel, see the Excel Graphing Exercise on the Chemistry 11 Laboratory Experiments web site.
Now that you have the equation relating position to wavelength for your spectroscope, you can use it to convert any position measured on your spectroscope into a wavelength. Thus, you can now measure wavelengths from any source of light by first measuring their position on your spectroscope and then using your graph to convert this position into a wavelength.
Be careful not to move your meter sticks or your diffraction grating! If the relative positions of these items is changed, the calibration line and its equation will no longer be accurate.
Part B: The Spectrum of a Polyelectronic Element
- Choose another discharge tube from the boxes provided. Do not choose hydrogen because you will use this tube in the next part of the experiment. Write the name of the element you chose above Table 2 on the data sheet. While the power supply is unplugged, remove the mercury discharge tube, mount the new tube in the power supply, then plug it in and turn it on.
- Use Table 2 to record the color of the five brightest spectral lines you see and their corresponding positions on the spectroscope. When you have finished, turn off your power supply and then use your calibration equation to determine the wavelengths of the lines you saw.
- Use one of the lab computers to go to http://physics.nist.gov/PhysRefData/...ement_name.htm . Select the name of the element you chose, then click on the box labeled “Strong Lines”. Scan the wavelength column for the wavelengths you measured to see if you can find any close matches. Note that the only lines you will have been likely to observe are those with the greatest intensities (see the Intensities column next to the wavelengths).
- In Table 2, recored the tabulated wavelength for the intense line nearest to each wavelength you observed.
- Calculate the % error for each of your measured wavelengths.
Part C: The Spectrum of a Single Electron Atom: Hydrogen
- With the power supply unplugged, place a hydrogen tube in your power supply then plug it in and turn it on.
- Record the colors and positions of the lines you see in Table 3. When you have finished, use your calibration equation to determine the wavelengths of the lines you saw.
- Using the information discussed earlier regarding Bohr’s theory, calculate the wavelengths of the first six lines in the Balmer series. Record your calculated results in Table 4.
- Compare your calculated wavelengths with your measured wavelengths. See if you can determine which electronic transition (from n = ? → n = 2) is responsible for each of the lines you saw in the hydrogen spectrum. Record your results in Table 5 and calculate your percent error for each line.
- Assume that the calculated wavelength is the actual wavelength:
\[ \text{% error} = \frac{|\text{observed wavelength} - \text{actual wavelength|}}{\text{actual wavelength}} \times 100\]
Pre-laboratory Assignment: Atomic Spectra
- Calculate the energy of the n=1 level for an electron in a hydrogen atom.
- Calculate the energy of the n=2 level for an electron in a hydrogen atom.
- Calculate the energy change when an electron in a hydrogen atom moves from n=2 to n=1.
- Calculate the wavelength of the light that an electron in a hydrogen atom would emit if it moved from n=2 to n=1.
- We can't see the light emitted by hydrogen atoms when the electrons move from any upper level to the n=1 level. Why not?
Lab Report: Atomic Spectra
Part a: calibration of the spectroscope.
Table 1: Emission Spectrum of Mercury
- Equation of best fit line from Excel (\(\lambda = mx + b\), where \(x\) = position):
- R 2 :_________________
Your instructor may ask you to attach a copy of your graph. Check with your instructor to see if this is required.
Part B: Spectrum of a Polyelectronic Element
Table 2: Atomic Spectrum of __________
Part C: Spectrum of a Single Electron Element: Hydrogen
Table 3: Atomic Spectrum of Hydrogen
Table 4: The First Four Lines of the Balmer Series
Table 5: Comparison of Observed and Theoretical Results for Hydrogen
- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information
PRE LAB 4 - pre lab report for atomic and molecular spectroscopy experiment
General chemistry i laboratory (chem 123l), the university of new mexico, recommended for you, students also viewed.
- Chapter 1 HW Questions
- Gandhi Essays Gandhi Essays
- Sports Psychology Essay
- Reality Television Essay
- Communications Technology Essay
- Performance Enhancing Drugs Essay
Related documents
- Racist Essay Racist Essay
- Name Of Book In Essay
- Reconstruction After The Civil War Essay
- Property Outline
- Neuromuscular Drugs - Dopamine Receptor Agonist
- Final Exam All Notes&Quizes
Preview text
Atomic and molecular spectroscopy.
Experiment 4
November 11, 2020
Bassam abou yassine
Chem 1215L-
Hypothesis:
Elements can be identified by the color results of a firework. By using a spectroscope when the element is producing its display colors through burning, the wavelength of the emissions can be determined. Each wavelength correlates to a specific color emission, so if we know the color emission, we can find the unknown element. By comparing this wavelength to the already known, we can identify what element is being burned.
The absorption and fluorescence spectrum can be used to assist in the identification of unknown dyes by providing graphs of each dye. The peak of these wavelengths from the spectrums can then be compared to the wavelengths and peaks of an unknown to identify them.
The difference between atomic emission and molecular emission can be determined by using a spectroscope to look the emissions of a gas lamp. From there a calibration curve is computed to find the wavelengths of the light emissions of an unknown, using the equation produced. These lengths are compared to the known atomic spectrum to label the unknown gas.
Lab Safety:
Chemical Name: Water Molecular Formula: H 2 O
Molecular Mass: 18 g/mol Concentration/Amount Used: 10 mL
Hazards: Generally expected to be non-hazardous. No harm to body.
Precautions & Protections: Protection not required. Always follow lab dress code.
Disposal & Spill Clean-Up: Can be poured down drain, if spilled clean with paper towels.
Chemical Name: Magnesium Chloride Molecular Formula: MgCl 2
Molecular Mass: 95 g/mol Concentration/Amount Used: No data
Hazards: Harmful if swallowed. Do not come into contact, or inhalation: May cause respiratory tract irritation
Precautions & Protections: Handle with gloves and lab goggles. Always follow lab dress code. If contact is made, use safety shower for 10-15 minutes and notify TA.
Precautions & Protections: Always follow lab dress code and wear goggles. Keep in properly labeled container. If contact occurs on skin or eye rinse in safety shower station for 10-15 minutes, notify TA immediately.
Disposal & Spill Clean-Up: If spilled, use inert absorption to minimize. Use water and paper towel once minimized. Keep in labeled container and dispose in proper waste container.
Chemical Name: Lithium Chloride Molecular Formula: LiCl
Molecular Mass: 32 g/mol Concentration/Amount Used: No Data
Hazards: Harmful if swallowed. May cause irritation to eyes. Do not ingest. May form combustible dust composites.
Disposal & Spill Clean-Up: Follow lab protocol. Once spill is neutralized wipe are with water and paper towels. Dispose of waste in labeled, designated waste container.
Chemical Name: Zinc Chloride Molecular Formula: ZnCl 2
Molecular Mass: 136 g/mol Concentration/Amount Used: No Data
Hazards: Harmful if swallowed. May cause irritation to skin and eyes. Acute toxicity. Damaging to the environment, specifically aquatic life. May cause severe eye damage. May form combustible dust.
Precautions & Protections: Keep in labeled container. Adhere to lab dress code. Always wear safety goggles. Wear protective gloves/face shield when handling. If contact occurs, use safety shower for 10-15 minutes, and notify TA immediately. Contact medical aid.
Disposal & Spill Clean-Up: If spilled, use inert absorption**.** Once spill is neutralized, clean area with water and paper towels. Dispose of waste in designated waste container.
Chemical Name: Strontium Chloride Molecular Formula: SrCl 2
Molecular Mass: 158 g/mol Concentration/Amount Used: No Data
Hazards: Causes serious eye damage. May cause respiratory irritation.
Precautions & Protections: Always follow lab dress code and wear goggles. Wear protective gloves and clothes when handling. Keep in properly labeled container. If contact occurs on skin or eye rinse in safety shower station for 10-15 minutes, notify TA immediately.
Disposal & Spill Clean-Up: Once spill is neutralized, clean area with water and paper towels. Dispose of waste in designated waste container.
Chemical Name: Sulfur tetrachloride Molecular Formula: SCl 4
Molecular Mass: 173 g/mol Concentration/Amount Used: No Data
Hazards: Causes severe skin burns and eye damage. May cause respiratory irritation. Do not inhale of ingest. Toxic to respiratory system.
Precautions & Protections: If contact occurs on skin or eye rinse in safety shower station for 10-15 minutes, notify TA immediately. Always follow lab dress code and wear goggles. Keep in properly labeled container. If ingested do not induce vomiting, call poison control.
Disposal & Spill Clean-Up: Once spill is neutralized, clean area with water and paper towels. Dispose of waste in designated waste container. Ensure adequate ventilation.
Chemical Name: Copper (II) Chloride Molecular Formula: CuCl 2
Molecular Mass: 134 g/mol Concentration/Amount Used: No Data
Hazards: Harmful if swallowed and if contact occurs with skin. Causes skin irritation and serious eye damage. Very toxic to aquatic life. Do not ingest.
Precautions & Protections: Always follow lab dress code and wear goggles. Keep in properly labeled container. If contact occurs on skin or eye rinse in safety shower station for 10-15 minutes, notify TA immediately. If ingested call poison control.
Disposal & Spill Clean-Up: Once spill is neutralized, clean area with water and paper towels. Dispose of waste in designated waste container. Avoid dust formation.
Chemical Name: Manganese(II) chloride Molecular Formula: MnCl 2
Molecular Mass: 125/mol Concentration/Amount Used: No Data
Calibrate spectroscope using a mercury lamp. Go to gas lamp under fume hood and hold it up Take the spectroscope and point it directly to the light at the bulb of the lamp. Record the numbers that correlate to the bands of light in the spectroscope and the color they correspond with. Using excel, select the table and plot the measurements you recorded as the x values. For the y values, use the known values of the correlating colors. Draw a trend line with equation shown on graph which will be the calibration curve of the spectroscope. Experiment 2: Gather materials: a. Goggles b. Cuvettes c. Pipette d. SpectroVis Plus Get two cuvettes and take them to the fume hood. Dilute the given unknown dyes using water Add 2-3 drops of dye to cuvette Fill rest with water Return to seat and power up SpectroVis Plus. On computer click “Experiment” and go to calibrate and click “SpectroVis” Wait until warm up time is complete then click “Calibration” When calibration is complete, take out cuvette. Place a cuvette with a dye in it into the slot and make sure the collection is on “Absorbance” Begin data collection and collect data until you see a distinct peak in the graph and record it. Repeat those steps with the others dye collected. Once all peaks for dyes are recorded dispose of chemicals in the waste container. Retrieve unknown dye and record it. Repeat steps with unknown dye and record the peaks. Compare unknown wavelengths to those gathered with the known chemicals. Determine the unknown dye. Rinse empty cuvettes with DI water and return to proper storage. Make sure your lab area is clean. Experiment 3: Gather materials: Goggles Spectroscope Bunsen Burner Chemical Humidifier Calibrate spectroscope using a mercury lamp. Burn the chemicals, and record the emission. When the chemical is burning point spectroscope directly at the flame. Record the length from the spectroscope.
Plug this length into the equation as your x value from procedure #1. Record final wavelength from the equation. Repeat steps this procedure of experiment 2 for all chemicals burned. Compare the color emitted and the wavelength calculated to the known emission spectra of the ten possible chemicals your TA has provided you with. Determine the types of elements that were burned through this comparison.
- Multiple Choice
Course : General Chemistry I Laboratory (CHEM 123L)
University : the university of new mexico.

- Discover more from: General Chemistry I Laboratory CHEM 123L The University of New Mexico 10 Documents Go to course
- More from: General Chemistry I Laboratory CHEM 123L The University of New Mexico 10 Documents Go to course
- More from: MH MH Mahdi Hossaini 999+ impact 999+ The University of New Mexico Discover more
Bloomsburg Instrumentation 2024 documentation

Atomic Spectroscopy
Atomic spectroscopy #.
Atomic spectroscopy is used for qualitative identification and quantitative analysis of trace metals in all types of materials and solutions. In atomic absorption spectroscopy (AA), measurement is of radiation absorbed by an unexcited vapour phase atom. In an atomic emission (AE) spectroscopy, the measurement is of energy emitted when thermally excited vapour phase atoms return to the ground state. In this laboratory, a flame will be used to desolvate, vaporize and atomize the samples, and also excite in the AE experiment.
Atomic Absorption Spectroscopy #
Analysis of a sample by atomic absorption requires it to be heated to a high temperature to evaporate the solvent, vaporize the sample, and dissociate the chemical bonds to release free metal atoms. These metals will then absorb light characteristic of the individual species. Atomic spectra consist of a series of narrow bands. The spectroscopist must choose the band with the proper sensitivity and lack of interfering radiation. Each element will be excited by a light source unique for that element. These sources must produce a narrow band of light of adequate intensity and stability over the course of the experiment. Because a monochromator can not produce a band any where near the width of an atomic absorption band (2 to 4 pm), the lamp must provide the bandwidth. Typical sources are hollow cathode lamps which will have a cathode made of the metal of interest. The lamps are filled with an inert gas (argon or neon) which ionizes when a voltage is passed between the cathode and an anode. These ions bombard the cathode causing cathodic atoms to be sputtered and a metal vapor fills the tube. Collisions between the metal vapor and the gas atoms causes excitation. Subsequent return to ground state is accompanied by a photon emission of the perfect frequency for the analyte to absorb. A hollow cathode lamp is shown in Fig. 3 .
Fig. 3 Schematic of a hollow cathode lamp for AA. The cathode is made of metal being studied. #
The hollow cathode lamp is the first part of the optical path of an atomic absorption instrument ( Fig. 4 ). The design shown here is a single beam system. The light emitted by the lamp passes through the atomic reservoir, a flame in this example, through a monochromator, and on to a photodetector. The monochromator is placed after the atomic reservoir to remove thermal emission by the reservoir itself. To remove any atomic emission caused by thermal excitation of the analyte, the lamp is frequently pulsed at 60 Hz and only 60 Hz modulated signal is recorded by the detector. The atomic reservoir is fed by a nebulizer system. The nebulizer operates by the gas flows of oxidant (air) and fuel (acetylene) feeding the flame. These gases pass a small orifice inside the nebulizer causing solution to be pulled up a capillary tube and into the nebulizer body as a mist. The mist is mixed with the gases and expelled out the burner head for combustion. The rate of aspiration of the material (affected by gas pressures, nebulizer efficiency, viscosity), choice of solvent (organics improve the nebulization), concentration (higher concentrations increase viscosities), surface tension of the solvent (a lower surface tension leads to better droplet formation and higher efficiency of sample reaching the flame), and the temperature will control the amount of sample actually available in the atomic reservoir for the analysis.
Fig. 4 Schematic of single beam atomic absorption spectrometer. #
Quantitative analysis is done by Beer’s law where absorbance is proportional to concentration. Sensitivity and detection limit are two terms used to describe the quantitative performance of an instrument. Sensitivity (for atomic absorption) is defined as the concentration of analyte that will produce a percent absorption of 1% (or 99% T or an absorbance of 0.0044.) It is expressed in terms of μg/mL per 1% absorption. The detection limit is the concentration of analyte which gives a signal twice the noise level of the background. If the detection limit is small compared to sensitivity, then small concentrations can be measured with little error. If the detection limit is equal to or larger than sensitivity, then much of the signal is actually noise and error in measuring that species will be large.
To properly quantitate, the standards used for calibration should be made in a similar matrix to that of the sample solutions. For example, if sera samples are to be measured for calcium, the calibration standards should be made to simulate serum. When a complex matrix is presented, the technique of standard additions should be employed. In these experiments, several small amounts of the standard solution are added to a large amount of the sample solution to preserve the matrix. A plot of absorption vs added standard concentration is plotted and the value extrapolated from the \(x\) -axis ( Fig. 5 ). In order to properly extrapolate, care must be taken involving the linear range for each species at each atomic line. Linearity decreases above 50 μg/mL for most species.
Fig. 5 Standard additions plot showing extrapolation to unknown concentration. #
Atomic Emission Spectroscopy #
The apparatus and theory are the same except that the excitation is thermal not by photon absorption. The same apparatus as AA is used with the exception that the hollow cathode lamp is not used.
Experimental #
Agilent 240FS AA in Flame configuration
Hollow cathode lamps (Cu, Ca)
Source of acetylene (over 100 psi) and air (over 60 psi)
Volumetric flasks, beakers
1000 ppm stock solution of copper and calcium
10000 ppm potassium
Ethanol or propanol
Concentrated Phosphoric acid
Lanthanum nitrate, 10% aqueous solution
Procedures #
This experiment was written for a PE3100 system. All early parts may not be possible with the new Thermo [Agilent dam] system with that d&*% computer control. cph
Instrument Parameters #
Prepare the instrument according to the instruction manual using a copper hollow cathode lamp. Turn on the instrument and set the proper current, wavelength, and slit width for copper (usually 324.7 nm with 0.7 nm slit for air-acetylene). Allow the tube to warm as you align the beam with the burner. Tape a ruler to the back wall of the burner chamber so that you can read the height of the burner in the next part. Turn on the gases (air first , then acetylene).
Burner Height #
Adjust the gas pressures to give a pale yellow flame, then turn up the acetylene to give a strong yellow flame. Such a flame is called fuel rich and is yellow due to light reflecting off of unburned carbon particles. (In a lean flame, there is excess oxygen present and the flame is blue.) Aspirate a 5 ppm copper solution. Adjust the monochromator to give a maximum signal to set the monochromator to the copper frequency. Raise the burner with the height adjustment knob until the light beam just passes over the tip of the burner. Use the ruler to measure the height above the burner head. Zero the instrument using distilled water and measure the absorbance of the 5 ppm copper solution as you lower the burner. Use a fairly large integration time to minimize noise and average several signals at each height. Plot absorbance vs. height of observation in the flame and select the optimum burner height from the graph.
Fuel/Air Ratio #
With the air pressure constant, record the absorbance of the 5 ppm copper solution as the acetylene pressure is adjusted in increments from a fuel-rich (yellow) to a fuel-lean (blue) flame. When the pressure can not uphold combustion — you have gone far enough. Plot absorbance vs. acetylene pressure (psi) to determine the optimum pressure for maximum sensitivity of copper. With the optimum acetylene pressure, adjust the air pressure from a fuel-rich to a fuel-lean flame as absorbance is measured for the 5 ppm solution. Plot to determine the optimum air pressure.
Treatment of Data #
Show all three plots in your report. Indicate whether the flame is rich, stoichiometric, or lean.
Quantitative Analysis of Copper #
Calibration curve #.
From the 100-ppm Cu stock solution, prepare 1, 2.5, 5, 7.5, and 10 ppm Cu in 100-mL volumetric flasks. Store in plastic beakers to avoid contamination from glass. Zero the instrument using distilled water then aspirate and record several absorbances for each solution using water in between each sample. Similarly, record the absorbance of an unknown.
Standard Addition #
To 10.0 mL aliquots of the unknown placed in clean, dry beakers, add 0.2 mL, 0.4 mL, 0.6 mL, 0.8 mL, and 1.0 mL of 100-ppm Cu stock solution. Zero the instrument with distilled water and measure the absorbance of unknown and unknown with various amounts of added copper.
Construct a calibration curve by plotting absorbance vs. concentration (in ppm). Determine the unknown concentration from the plot. If the plot is nonlinear, explain the causes of nonlinearity. Calculate the analytical sensitivity for copper, i.e., the concentration which will give an absorbance of 0.0044. Also calculate its detection limit. Lastly, construct a standard additions plot. Don’t forget to correct for volumes. Extrapolate and report the concentration of copper in the unknown. How does this value compare to the calibration curve? How do the slopes compare? You may wish to refer to Instrument Calibration as you decide which answer is better!
Effect of Solvents #
Miscible organic solvents #.
Prepare solutions 1, 2.5, and 5 ppm Cu(II) in the following three solvent systems by appropriate dilution of the 100 ppm Cu(II) stock solution: (1) water, (2) 20% ethanol or propanol in water, and (3) 50% ethanol or propanol in water.
Use a wavelength of 324.7 nm and a 0.2 nm slit width. Optimize the instrument with the 5 ppm aqueous Cu solution. Aspirate and read the aqueous solutions using water as a reference. Aspirate and read the 20% alcohol solutions then readjust the flame to remove the yellow color and read the 20% alcohol solutions again. Return the flame to the same acetylene pressure as used for the water samples and aspirate and read the 50% alcohol solutions. Again readjust the flame to remove the yellow and read the 50% alcohol solutions again.
On a single axes, plot all the calibration curves. Which solvent system exhibits the best sensitivity for copper? Explain. Why are we not concerned with best linearity? Explain.
Interferences #
Ionization interference in calcium determinations #.
Ions of an element absorb light at a different frequency than its neutral atoms. Addition of an excess of a more easily ionized element will produce a large number of free electrons in the flame and shift the ionization equilibrium so that the element of interest is insignificantly ionized.
Prepare (a) calcium solutions of 8 ppm and 200 ppm containing 0, 1, 10, 100, or 1000 ppm potassium and (b) calcium solutions of 8 and 200 ppm without potassium.
Use a calcium hollow cathode lamp, a wavelength of 422.7 nm and a slit of 0.2 nm. A nitrous oxide-acetylene flame would give a more noticeable effect but the air-acetylene flame will suffice.
For the 8 ppm solutions, set the aspiration at a fairly fast rate. Zero the instrument using distilled water and record the absorbance of each solution. With the 200 ppm samples, set the aspiration to a much slower rate and using distilled water as the blank, record each solutions absorbance. In either case, measure aspiration rate.
Plot absorbance vs concentration of potassium. From the plots, determine the minimum amount of potassium needed to overcome calcium’s ionization effects. Explain!
Phosphate Interference #
Atomic absorption signals for alkaline earth elements are depressed by the presence of anions such as phosphate which form relatively nonvolatile compounds (like calcium pyrophosphate) in the flame. This type of chemical interference can be minimized by solvents which cause smaller particles to form in the nebulizer. These smaller particles are more easily vaporized. Hotter flames can also produce more vaporization (and often ionization). In addition, an excess of a chemical releasing agent, a species which has a larger equilibrium constant with phosphate may be used.
Prepare solutions of 200 ppm Ca in 50 mL of 95% ethanol plus 0, 0.1, 0.3, 1.0, 3.0, or 10.0 mL of concentrated phosphoric acid (85% acid, 14.7 M) with dilution to 100 mL with water. Flames of nitrous oxide-acetylene (2990 K) and air-acetylene (~2500 K) are used. Use a wavelength of 422.7 nm and a 0.2 nm slit. Use a blank with an appropriate amount of phosphoric acid and ethanol to set the instrument to zero.
Also prepare 20 ppm calcium with 0, 0.1, 0.3, 1.0, 3.0, and 10.0 mL of concentrated phosphoric acid and containing 1% lanthanum or strontium with dilution to 100 mL. Use an air-acetylene flame with the conditions as above.
For the ethanol solvents, plot the results for both burners on the same axis. Plot apparent calcium concentration vs. Log of the mole ratio (Ca/PO 4 ) for both sets of data. Explain how the signal for 200 ppm Ca varies with presence of phosphate, e.g., where does Ca go? For the lanthanum, plot absorbance vs phosphate concentration and explain the curve shape, e.g., why does Ca come back?
Turn off the acetylene at the main tank valve and allow the line to purge free and the flame to go out. Then, shut off the air and bleed the line of excess pressure so as to not damage instrument components. Set the lamp current to zero and turn off the instrument.
Questions #
A premix or laminar flow burner does not introduce all the material into the flame and the larger droplets are drained off to waste. How is air prevented from backing up into the burner and possibly causing an explosion?
What other anions are likely to cause low responses for alkaline earth samples? Explain.
In atomic absorption spectroscopy why is the monochromator located after the sample compartment (the flame) rather than before as in the case of a UV-visible absorption spectrophotometer?
What is beam modulation and why is it used in atomic absorption spectroscopy?
Why are atomic absorption lines so sharp compared to the absorption lines of a molecule dissolved in solution?
How does the detection limit of atomic absorption compare with flame emission? Give some specific examples.

IMAGES
COMMENTS
SÁ§Â%"é¬ º Ç þôlÇõx}~ÿïÛ´ÿ ç¼ ÿ[gR µ¼³ -!KÓ I&Ðv¦% #[×X ,¹’ÌRÊÿß›ZÚ™ŠvÎK ¯¼ ¡à”Wq³ oz=?Ty*¡] =HÍ )õ1@¶Î!Å™ûî{ÿׯ_ 4 @ ²û Jî–&PaR¢Ô3ÎiçÅ:¦‰«¼r\ìÓºPp`Ë!¦ÅÎû¥ CuYçÞMˆˆ@0i·ˆ9»²ý-£; ǯ,ë l K;wÛ=µ¶û„ € ÀmÒ繶yg² dø% 1b» M«“t]–ÿ !¦e† XîsÌÿJ _¶”“¶ ëù 1³‹W ...
In this experiment and the next, you will see how spectroscopy, the measurement of light absorption and emission by atoms and molecules, can yield important information about atomic and molecular structure. In Part A of this experiment, you will study the energy levels of the hydrogen atom by observing
Atomic and Molecular Spectroscopy. Experiment 4. November 11, 2020. Bassam abou yassine. Chem 1215L-Hypothesis: Elements can be identified by the color results of a firework. By using a spectroscope when the element is producing its display colors through burning, the wavelength of the emissions can be determined.
In this the experiment you will observe three different spectral lines and measure the angle of diffraction θ of each line. The relationship between the wavelength λ, the grating spacing d and the angle θ is mλ= dsinθ m=0,±1,±2,... (1) where m is the order of diffraction which locates the maxima. In this experiment you can only see
Atomic Spectroscopy Experiment objectives: test adiffractiongrating-basedspectrometer, study the energyspectrum ofatomic hydrogen (H) and a hydrogen-like atomic sodium (Na), determine values of quantum defects of low angular momentum states of Na, and measure fine splitting using Na yellow doublet. History
Atomic Spectroscopy Experiment objectives: test a di raction grating-based spectrometer, study the energy spectrum of atomic hydrogen (H) and a hydrogen-like atomic sodium (Na), determine values of quantum defects of low angular momentum states of Na, and measure ne splitting using Na yellow doublet. History
technique known as spectroscopy. This technique allows us to investigate the material composition of objects ranging from very small samples to distant stars. In this lab you will use a diffraction based spectrometer to measure the emission spectrum of hydrogen and use the Rydberg formula to match each line in the spectrum with an atomic ...
Chemistry 1A Experiment 8: Atomic Spectroscopy CSUS Rev. F11 Page 3 of 5 EXPERIMENTAL: Part 1 Line spectra of elements Working in groups of three or four, view the emission spectra from the discharge tubes set up in the room containing hydrogen and an additional element using a spectroscope.
In an atomic emission (AE) spectroscopy, the measurement is of energy emitted when thermally excited vapour phase atoms return to the ground state. In this laboratory, a flame will be used to desolvate, vaporize and atomize the samples, and also excite in the AE experiment. Atomic Absorption Spectroscopy#
Atomic Spectroscopy Experiment objectives: identify the chemical composition of some mystery discharge lamps by analyzing their visible spectra. History The observation of discrete lines in the emission spectra of atomic gases played fundamental role in developing of the quantum mechanics and gave an important insight into the quantum nature of ...