- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board

Study: Your Voice May Reveal If You Have Type 2 Diabetes
Luis Alvarez/Getty
Key Takeaways
- A new artificial intelligence (AI) model may be able to tell a person has type 2 diabetes with just short clips of the person’s voice.
- The researchers say that people who have diabetes can develop diabetic neuropathy or nerve damage, which can affect their vocal function, potentially leading to changes in the pitch and strength of their voice.
- Screening and diagnosis for type 2 diabetes currently involves questionnaires, blood tests, and glucose tolerance tests.
The most common and accurate way to diagnose diabetes, including prediabetes and type 2 , is through blood tests. However, a new study suggests that type 2 diabetes could also be detected in a person’s voice.
Researchers from Klick Labs have created an artificial intelligence (AI) tool that can determine if a person has type 2 diabetes using just six to 10 seconds of their voice, combined with basic health information like age, sex, height, and weight. The AI model was 89% accurate in diagnosing type 2 diabetes in women and 86% accurate in men.
“Our vision is to create a screening method that is easy, convenient, and alleviates the burden and associated costs with the current blood tests,” Yan Fossat, the Vice President of Klick Labs and principal investigator of the study, told Verywell. “Voice-based screening is extremely accessible compared to the standard blood tests. A voice screening tool could be implemented outside of labs or doctors’ offices, and using people’s cell phones.”
Here’s what else you need to know about the study, including how diabetes can affect a person’s voice and whether experts think an AI model could become a new screening tool for diabetes.
Listening for Clues
For the study, Fossat and colleagues recruited 267 participants in India. They noted that 192 of the participants (79 women and 113 men) did not have diabetes. The other 75 (18 women and 57 men) had previously been diagnosed with diabetes.
All the participants used a smartphone app to record themselves saying a six- to 10-second fixed phrase up to six times a day for two weeks. The researchers analyzed the 18,465 recordings that were collected to listen for 14 “acoustic characteristics.” The idea was that these sounds could show vocal differences between people with type 2 diabetes and people who didn’t have the condition.
The researchers also considered other vocal features—like changes in pitch, strength, and intensity—that the human ear can’t pick up on. With help from a technique called signal processing and voice analysis software, the researchers were able to note subtle changes in the voices of people with type 2 diabetes. They used this data to train a machine learning model to be able to make predictions about type 2 diabetes based on a voice clip.
Jaycee Kaufman, a research scientist at Klick Labs and first author of the study, told Verywell that the team identified differences in vocal features between males and females with type 2 diabetes.
For example, Kaufman said voice pitch and variability of pitch were affected in women, while strength or intensity of the voice and variety of strength were affected in men.
“We believe this difference may stem from the fact that men and women experience the complications of type 2 diabetes differently, which ultimately impacts the voice differently,” said Kaufman. “Specifically, men may experience more muscle weakness associated with type 2 diabetes, whereas women may experience more edema.”
According to Kaufman, other researchers have used voice to predict neurodegenerative diseases like Alzheimer’s and Parkinson’s .
“The production of voice is a complicated process that involves the combined effects of the circulatory system, respiratory system, muscular system, nervous system, and other systems in the body,” said Kaufman. “Anything that affects these systems may have an effect on the voice, which was the motivation for this work.”
Why Diabetes Can Affect Your Voice
People living with diabetes can develop nerve damage called diabetic neuropathy . In some cases, nerve damage leads to voice problems like bilateral vocal fold paralysis . In this condition, both vocal cords and the voice box can become partially or completely paralyzed.
Type 2 diabetes is also associated with other health problems, such as muscle weakness and swelling . According to Kaufman, previous studies have shown that the swelling can affect the voice.
“Peripheral neuropathy may damage the nerves in the larynx, resulting in hoarseness or vocal strain, and muscle weakness may be apparent in the muscle in the vocal cords or respiratory system,” said Kaufman. “In addition, the swelling associated with edema may affect the elastic and vibrational qualities of the vocal cords, which could affect the pitch.”
Giving Undiagnosed Diabetes a Voice
The study is one of the first to show how AI could help detect chronic disease, Steven Malin, PhD, FACSM , an associate professor of metabolism and endocrinology in the Department of Kinesiology and Health at Rutgers University, told Verywell. Malin was not involved in the study.
However, Malin said that without more research, it’s too early to say whether the technology will make diagnosis faster, cheaper, or more accessible for patients. While diagnosing diabetes from a voice clip is intriguing, more studies need to be done to see if the technology would work for people of different races and ages .
That said, there’s promise in having another screening tool in our arsenal.
“Even if these have ‘false-positive’ assessments, the identification of people at risk can afford people the opportunity to make behavioral changes that mitigate risk,” Malin said.
Jaycee Kaufman
We believe this difference may stem from the fact that men and women experience the complications of type 2 diabetes differently, which ultimately impacts the voice differently.
Other experts who were not involved in the study said that the findings come at an important time, as the number of people with diabetes is rising and we need better ways to screen for it. According to the Centers for Disease Control and Prevention Diabetes Statistics Report, 14.7% of all adults age 18 or older in the United States had diabetes in 2019. And for 3.4% of the U.S. population, diabetes went undiagnosed.
“With increasing prevalence of diabetes, there is a clear need to increase screening in order to allow earlier detection of diabetes, and thus, diagnosis and treatment,” Priya Jaisinghani, MD , an endocrinologist and obesity medicine specialist at NYU Langone Health, told Verywell.
Fossat said that a non-intrusive and readily available tool like the AI-voice method they’ve come up with has the potential to screen a lot of people and could be especially useful for patients living in remote areas. More screenings could help reduce the number of people living with undiagnosed diabetes.
“We believe screening performed on a smartphone would be more accessible than a blood test and could assist in screening populations who may have more difficulties accessing healthcare,” said Fossat. “Furthermore, a mobile application for screening has the potential to reach vast amounts of the at-risk population, potentially aiding millions of people in receiving a type 2 diabetes diagnosis.”
How Is Diabetes Currently Diagnosed?
Providers can use different tests to detect and diagnose diabetes. Jaisinghani said these tests usually involve measuring blood glucose levels, which help show if someone has diabetes as well as what type they have. Tests like blood glucose (sugar), glucose tolerance, and hemoglobin A1C can all be used to find out if someone has diabetes.
Would Voice-Based Diabetes Tests Be Accurate on Their Own?
Jaisinghani said other factors could affect the accuracy of a diagnostic test that relies on voice or voice recordings, such as voice changes that stem from inflammatory and infectious disorders, neurological diseases such as myasthenia gravis, nerve injury leading to vocal cord paralysis, irritation from smoking or acid reflux, psychological or somatic conditions, and vocal stress.
“There also may be change to voice that needs to be further investigated or distinguished across age, gender, ethnicity, exposure to environmental factors, and socioeconomic demographics through further testing,” said Jaisinghani.
We believe screening performed on a smartphone would be more accessible than a blood test and could assist in screening populations who may have more difficulties accessing healthcare.
Kaufman added that it is reasonable to consider that vocal damage could affect the test’s accuracy.
“Among our next steps, we plan to explore the effect of sickness, smoking, and vocal damage on the test’s efficacy,” she said.
Since the research was based on a limited sample of people from India, Malin said that additional studies in different countries and with a diverse group of participants would be needed, especially since diseases differ across race and ethnicity.
According to Malin, another limitation of the study is that the data came from non-smokers, so it’s not generalizable to people who smoke. The data also wouldn’t apply to people who have changes in their speech from other factors, like neurological or speech disorders.
What the Researchers Are Planning Next
Experts agree that more research with larger sample sizes that are more generalizable to different populations will need to be done before an AI voice test for diabetes could be patient-ready.
Jaisinghani said it will also be important to identify how factors like how long a person has had diabetes, as well as the complications and comorbidities of the disease, affect the voice. From there, researchers will have to determine how the factors could be useful for screening people for diabetes.
Fossat’s team said they plan to do follow-up studies to gauge the effectiveness of the technique within the next year. They want to recruit new people from different parts of the world with different demographic characteristics so they can validate and improve the current model. After the follow-up study, they want to look at how other factors like illness, smoking, and vocal damage would affect the test’s effectiveness.
Centers for Disease Control and Prevention. Diabetes tests .
National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes tests & diagnosis .
Kaufman J, Anirudh Thommandram, Fossat Y. Acoustic analysis and prediction of type 2 diabetes mellitus using smartphone-recorded voice segments . Mayo Clin Proc Digit Health . 2023;1(4):534-544. doi:10.1016/j.mcpdig.2023.08.005
Hajjar I, Okafor M, Choi JD, et al. Development of digital voice biomarkers and associations with cognition, cerebrospinal biomarkers, and neural representation in early Alzheimer's disease . Alzheimers Dement (Amst) . 2023;15(1):e12393. doi:10.1002/dad2.12393
Dao SVT, Yu Z, Tran LV, Phan PNK, Huynh TTM, Le TM. An analysis of vocal features for Parkinson's disease classification using evolutionary algorithms . Diagnostics (Basel) . 2022;12(8):1980. doi:10.3390/diagnostics12081980
Hamdan AL, Kurban Z, Azar ST. Prevalence of phonatory symptoms in patients with type 2 diabetes mellitus . Acta Diabetol . 2013;50(5):731-736. doi:10.1007/s00592-012-0392-3
Centers for Disease Control and Prevention. Prevalence of both diagnosed and undiagnosed diabetes .
By Alyssa Hui-Anderson Hui-Anderson is a health news writer and former TV news reporter. She was the 2020 recipient of the Midwest Broadcast Journalists Association Jack Shelley Award.
- News in brief
- Diabetes views
- Advocating for diabetes
- Caring for diabetes
- Living with diabetes
- Diabetes profiles
Search your topic
- Submit an article
- Middle East and North Africa
- North America and Caribbean
- South and Central America
- South-East Asia
- Western Pacific

Global perspectives on diabetes

- Visual storytelling
The impact of language in diabetes
Experts say words matter when speaking directly to people with diabetes. diabetes voice reached out to two individuals to express their views on the subject., share this:.
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)

Experts say words matter when speaking directly to people with diabetes. New guidance from two sources, Language Matters: Language and Diabetes (NHS, June 11, 2018) and the American Association of Diabetes Educators (AADE) and the American Diabetes Association (ADA) in The Use of Language in Diabetes Care and Education (October 17, 2017) recommend that healthcare providers should be attentive and respectful of words when discussing diabetes treatment and test results with patients in a way that encourages and motivates. The objective of each guidance is an effort to reduce any negativity in the language used by clinicians that might lead to unintentional bias or express negativity which can adversely affect the psychosocial well-being of the individual. According to an important paper from 2017, “For people with diabetes, language has an impact on motivation, behaviours, and outcomes” .
The language of diabetes and the stigma associated with words like control, non-compliant and diabetic (used as both a noun and an adjective) don’t only apply to the healthcare setting; they are often heard or seen in the home, office and more traditional or social media platforms. In light of this global discussion, Diabetes Voice reached out to two individuals to express their views on the subject. Betsy Rodríguez , who lives with diabetes, is a nurse, diabetes educator, national and international speaker on diabetes-related topics, bicultural specialist in health communication strategies, and author. She presently serves as a Senior Public Health Advisor in the Translation Health Education and Evaluation Branch in the Division of Diabetes Translation (DDT) at the Centers for Disease Control and Prevention (CDC). Paul Sandells was diagnosed with type 1 diabetes in 1984. He is a vlogger and lives in the United Kingdom. Each of our contributors represent perspectives that illuminate the impact of language in diabetes.

Why does language matter when we talk about diabetes?
Betsy Rodríguez
Have you ever been on the wrong side of a conversation at a clinic or a hospital, where the language used to describe your condition sounded critical and made you feel judged and even, depersonalized? I live with diabetes and I have been called a diabetic patient many times! At some point, you start asking yourself, since when did my full identity become diabetic patient instead of a person who has diabetes or a person living with diabetes.
There are many examples of this conundrum. Perhaps while searching online, you come across a website, talking about diabetic feet so now I am realizing that my feet are diabetic too! On the Internet, you are more likely to skip to the next website in your search results. However, in a clinic setting, you would likely: a) seek out another healthcare team or doctor or b) decide to stick with them and work to increase their awareness, helping them understand that when it comes to diabetes, language matters!
Words are powerful weapons that healthcare teams can utilize to empower people living with diabetes or demotivate them.
As a person with diabetes, I am not defined by my diagnosis. Language used in the community and in the healthcare system has been a battle that many people have been fighting for years. This is not only happening in the diabetes community; one example is the work of parents of children with Down syndrome and the Global Down Syndrome Foundation and their campaign Words Can Hurt. For people with Down syndrome and their families, the history of labels is not a pleasant one. People with Down syndrome were associated with words like idiot, moron, and imbecile by society and the medical profession. Today, these labels are considered politically incorrect, hurtful and dehumanizing.
In the diabetes community, the topic of why language matters for healthcare advisors, professionals and people with diabetes is connected to the nature of language in diabetes care, in the media, and the stigma associated with language when diabetes is the topic. In 2016, Diabetes Australia launched a position statement : A new language for diabetes: Improving communications with and about people with diabetes . ( Position Statement: A new language for diabetes , 2011) The aim of Australia’s position statement is to encourage greater awareness of the language surrounding diabetes, and to identify potential improvements. Diabetes Australia believes that optimal communication increases motivation, health and the well-being of people with diabetes. Furthermore, careless or negative language can be demotivating, is often inaccurate, and can be harmful. In 2017, The Use of Language in Diabetes Care and Educatio n was published. (Jane K. Dickinson, 2017) This publication opened many opportunities for the American Association of Diabetes Educators (AADE) to increase awareness about the way healthcare professionals talk to and about people with diabetes; how language plays an important role in engagement, conceptualization of diabetes and its management, treatment outcomes, and psychosocial wellbeing. A taskforce consisting of representatives from AADE and the American Diabetes Association convened to develop these guidelines using four guiding principles:
- Diabetes is a complex and challenging disease involving many factors and variables.
- Every member of the healthcare team can serve people with diabetes more effectively through a respectful, inclusive, and person-centered approach.
- Stigma that has historically been attached to a diagnosis of diabetes can contribute to stress and feeling of shame and judgement.
- Person-first, strengths-based, empowering language can improve communication and enhance motivation, health and well-being of people with diabetes.
As a person living with diabetes for more than 30 years, I can say that living with diabetes can be overwhelming at times. Like all chronic diseases, it affects every aspect of our daily routine. Diabetes management is not as simple as just taking a pill. It requires timing of meals, checking blood sugar and being vigilant about exercise, all in accordance with a personalized management plan developed in consultation with healthcare professionals.
The time has come to reflect on the language of diabetes and share insights with others. Messages of strength and hope will signify progress toward the goals of eradicating stigma and considering people first (Jane K. Dickinson, 2017).
Betsy Rodríguez, RN, MSN, DE is a nurse, diabetes educator, national and international speaker on diabetes-related topics, bicultural specialist in health communication strategies, and author, presently serves as a Senior Public Health Advisor in the Translation Health Education and Evaluation Branch in the Division of Diabetes Translation (DDT) at the Centers for Disease Control and Prevention (CDC). At CDC, Mrs. Rodriguez provides technical assistance and support to state grantees, national diabetes-related professional organizations, such as the American Diabetes Association (ADA) and the American Association of Diabetes Educators (AADE), as well as community-based organizations. At the international level, Mrs. Rodriguez provides technical assistance and support to the International Diabetes Federation, IDF SACA Region and is a member of the IDF Blue Circle Voices of Diabetes.

I am not offended by the word diabetic
Paul Sandells
While I support Language Matters and recognise that it is a force for the good, on the whole, it doesn’t really matter to somebody like me, a seasoned diabetic. How I choose to refer to my condition and my management of it is my own business. I’ve had type 1 diabetes (T1D) for almost 34 years. I’ve always considered myself to be a diabetic. It says so on my social media profiles and on the tattoo on my arm. I’m not offended by the word “diabetic”. I understand it doesn’t define me but it is a significant part of my life.
I believe the language used in a clinic environment is more significant but not to a very strict level. I want to know my HbA 1c isn’t good enough and if my healthcare professional (HCP) describes it as “poor” or “too high” then that is fine with me. I can take that information away and work on it, rather than sulk because I got a bad mark in class. I want my clinic team to be pro-active in helping me and others with diabetes, rather than walking on egg shells because of the terminology they might use.
Language matters to a point but my health matters far more than the words you use.
There is a fine line between offering constructive encouragement, even criticism and being downright offensive, though. I never want to be told that I’m a “bad diabetic” or even a “bad person with diabetes”. The latter actually sounds worse than the former to me! Neither do I want to be referred to as “non-compliant”. Those terms, amongst others, are very offensive when used by a HCP. In fact, unless used jokingly between friends, they are offensive if used by anybody. I realise that we are all different. We all have our line in the sand when it comes to what offends us and what doesn’t. Being offended and possibly extremely upset, following a clinic appointment, can never be a good thing when it comes to long term management of our diabetes.
We all respond differently to how a HCP approaches us, their body language, how they engage and how interested they are in our condition. If language does matter, then so do all those things. I am much more likely to be upset or offended if my HCP doesn’t look at me when I’m talking, isn’t open to my questions and doesn’t show a genuine interest in my care. I believe the vast majority do those things. I don’t want to be judged on my condition negatively because I live with T1D, my T1D, every single day. I’m not the same as the patient you saw earlier, with an HbA 1c of 6%. Don’t compare me to that person or any other diabetic. Comparison to “perfect” diabetics is what offends me far more than being labelled a diabetic and, to me, that’s what HCPs should be focusing on during appointments rather than worrying about how to tell me that my HbA 1c for the last 90 days is terrible. Work with me to manage my diabetes in the best possible way, based upon how I live and I’ll be a happy patient. Language matters to a point but my health matters far more than the words you use.
Paul Sandells is a type 1 diabetes vlogger from the United Kingdom. Diagnosed in 1984, he is a husband and father of two. @DiabeticDadUK

Elizabeth Snouffer is Editor of Diabetes Voice
You may also find these interesting
No related posts.
Do you have something to say?
Your thoughts and opinions matter to us., be the first to comment.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Evaluation and Management of Patients with Diabetes and Hearing Loss
Christopher spankovich , au.d., ph.d., m.p.h., krishna yerraguntla , ph.d..
- Author information
- Article notes
- Copyright and License information
Address for correspondence Christopher Spankovich, Au.D., Ph.D., M.P.H. Department of Otolaryngology and Communicative Sciences, University of Mississippi Medical Center, 2500 N. State St., Jackson, MS 39216, Email: [email protected]
Series information
The Role of Audiology in the Care of Persons with Diabetes
Guest Editor, Victor Bray, Ph.D.
Issue date 2019 Nov.
Diabetes mellitus is a significant risk factor for acquired hearing loss and tinnitus. Persons with diabetes (PWD) may present with hearing loss symptoms earlier in life than those without diabetes. Furthermore, diabetes may exacerbate risk for hearing loss related to noise exposure and ototoxic drugs. The purpose of this article is to provide recommendations for the prevention, screening, evaluation, and management of hearing loss in PWD.
Keywords: hearing loss, tinnitus, diabetes, prevention, screening, evaluation, management
The literature overall supports diabetes mellitus as a significant independent risk factor for hearing loss and tinnitus. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Diabetes can contribute to hearing loss affecting cochlear and neural elements 16 17 through numerous mechanisms, including microangiopathy, mitochondrial dysfunction, advance glycation end products/inflammation, and glutamate excitotoxicity. 7 18 19 20 21 22 23 24 25 The net result is increased risk for primarily acquired auditory sensory and neural pathology (i.e., sensorineural hearing loss). Increased risk for infection in persons with diabetes (PWD) also should alert the provider to risk for conductive pathologies of the external and middle ear. 26 In addition, medications for treatment of diabetes and diabetes-related symptoms may have ototoxic side effects including hearing loss and tinnitus (see DiSogra and Meech, 2019 in this edition).
Human and animal evidence suggests potential for damage along the cochlea length from basal to apical regions. 9 The distribution of changes along the cochlea may be related to the relative contributions of the array of mechanisms implicated in diabetes-related hearing loss. For example, microagniopathy may result in direct compromise of vascular supply to the inner ear; the apical region representing the most distal region of this supply may show early pathology. 9 27 28 29 30 On the contrary, elevated risk for noise-induced damage may underlie early changes observed in basal regions of the cochlea. 9 31
Potential for onset of hearing loss earlier in life, increased risk for other determinants of hearing loss (e.g., noise and ototoxic drugs), and risk of diabetes and hearing loss associated with balance dysfunction 32 33 and cognitive decline 34 35 support need for improved preventative approaches and guidelines for evaluation and management of diabetes-related hearing loss. The purpose of this manuscript is to present recommendations to inform prevention, screening, early identification, and management of hearing loss in PWD.
Prevention: Diabetes and Hearing Loss
Prevention includes a range of interventions aimed at reducing risk for compromised health. In general, the three categories of prevention considered include primary, secondary, and tertiary .
Primary prevention refers to prevention of disease or injury prior to onset. For hearing loss, an example of primary prevention would include regulations requiring use of hearing protection in the workplace or installing equipment with lower sound level output. These two examples represent active and passive strategies, respectively, with regard to the worker's behavior. In other words, use of hearing protection requires an active behavioral change among workers, while installation of equipment with lower sound emissions reduces risk without the worker having to make a behavioral choice.
Secondary prevention is aimed at slowing or reducing progression of disease or injury that has occurred. A critical concept in secondary prevention is early detection, which allows for timely intervention. For example, a patient needs to receive a medication with known ototoxic side effects to preserve functional status. Regular monitoring allows early identification of damage to allow implementation of potential otoprotective strategies, such as change in dosage level, change in schedule of drug, or providing hearing-assistive technology to prevent compromised communication. An essential element to secondary prevention of hearing loss is establishing baseline hearing status to allow sensitivity to detect change.
Tertiary prevention is consistent with what we commonly regard as “treatment or management.” The goal of tertiary prevention is to reduce impact of an ongoing disease or injury on function and quality of life. For example, a PWD has acquired hearing loss that is significantly impacting his or her interaction with family and friends leading to isolation and reduced quality of life. Fitting of appropriate amplification and aural rehabilitation recommendations can improve engagement with life and patient's overall well-being.
Primary, secondary, and tertiary prevention-based strategies can be applied at the individual, community, and system level. Furthermore, these concepts should be reinforced across prevention levels and at each patient interaction. Fig. 1 provides an overview of the prevention of hearing loss across categories and levels.
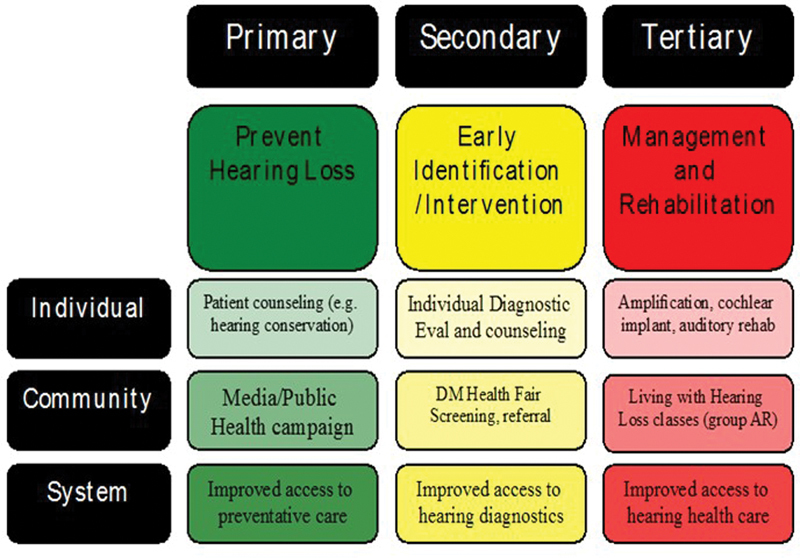
Primary, secondary, and tertiary prevention overview.
Improving Hearing Health Literacy
Health literacy and knowledge of determinants of disease/injury are critical to primary prevention. Primary prevention of hearing loss in PWD includes several strategies. The education of PWD with regard to elevated risk for hearing loss is a first step. Primary prevention strategies for diabetes-related hearing loss include the following:
Educating/counseling PWD on risk for hearing loss with diabetes, topics including:
Risk for earlier onset of age-related hearing loss.
Progressive nature of hearing loss due to uncontrolled diabetes.
Increased susceptibility to noise and drug-related hearing loss.
Importance of monitoring and early intervention.
Educating/counseling PWD on prevention of hearing loss, topics including:
Limiting noise exposure and use of hearing protection devices.
Awareness of ototoxic medications the patient may require.
Education/counseling PWD on early signs of hearing loss, signs including:
Fullness sensation.
Reduced ability to understand speech particularly in noisy environments.
Education/counseling PWD on importance of diabetes control (ABCs) and healthy lifestyle.
The ABCs refers to A1c, blood pressure, cholesterol, and smoking as factors the PWD can address to prevent low-risk individuals from moving to higher risk for diabetes-related complications and increased risk for hearing loss. 36 Lifestyle can drastically alter the health status of PWD. Lifestyle factors including diet, physical activity, and smoking are also related to hearing loss. 37 38 39 40 41 42
Recommendations for Screening and Diagnostic Evaluation
Screening recommendations for healthcare providers.
Early identification of changes in auditory function is necessary to provide timely intervention strategies. Screening PWD for hearing loss is important to successful prevention strategies. Screening options include pure-tone audiometry screening and otoacoustic emission (OAE) screening. Pure-tone audiometry screening involves presentation of tonal sounds at different frequencies important for speech understanding; the patient then reports sound detection. Testing is typically accomplished with the use of a pure-tone audiometer. Stimuli are presented to calibrated headphones or insert earphones typically at a single level (e.g., 25 dB HL) and at multiple frequencies important for speech understanding. The presence/absence of a detection response (e.g., raise hand) is interpreted as Pass/Refer. There are several online and smartphone-based applications for hearing screening; however, caution is warranted due to the variability of these applications that often lack appropriate calibration.
OAEs are an objective measure of cochlear function. OAE measurement involves administering a sound to the ear via a probe placed in the ear canal; the probe measures distortions and reflections generated in the cochlea that travel back to the external ear. OAEs do not require the patient to make a behavioral response. An OAE screening test involves measurement of responses from the cochlea and determination if the amplitude of the response meets some predetermined criteria for a Pass/Refer interpretation. OAEs are not a measure of hearing, but rather a measure of cochlear function. The presence of OAEs does not rule out mild, neural, or central hearing deficits and the absence of OAEs does not inform on the severity of the hearing loss.
Not all health care providers have access to the necessary equipment and these screening tests provide only limited insight on hearing status; referral to an audiologist may be a necessary step. Other screening tests include the whispered voice test, finger rub test, and watch tick test. The primary limitations of these screening measures are the broad variation in outcomes across examiners and lack of standardized procedures. 43 44 45
Some simple questions with regard to perceived hearing difficulty may provide insight on hearing status and help inform need for further evaluation. The following screening questions are recommended to determine need for further evaluation (these questions are based on those used in the Guide for Pharmacy, Podiatry, Optometry, and Dentistry [PPOD] for Diabetes Management):
Do you or your family perceive any change in your hearing?
Do you have hearing difficulty in quiet or noise?
Have you had your hearing tested in the past 2 years?
Do you know how diabetes can affect your hearing?
Do you know what to do if you perceive a change in hearing?
Do you know how to reduce your risk for hearing loss?
If the patient answers “Yes” to questions 1 or 2 or “No” to questions 3 through 6, it is recommended they be referred for a diagnostic audiological evaluation.
Numerous hearing screening options exist to inform next steps and need for more in-depth evaluation. Yet, screenings may miss subtle forms of hearing loss and limit the ability to establish a baseline assessment early in the diabetic disease process. Therefore, to establish baseline, to enable early identification, and to impart education on prevention strategies, we recommend a comprehensive audiological evaluation for all patients upon diagnosis of diabetes . If the patients report hearing difficulty or if they have not had their hearing tested in the past 2 years, they also should be referred.
Diagnostic Recommendations
A comprehensive audiological evaluation (pure-tone and speech audiometry) is recommended as a minimal test for determining the type and severity of hearing loss in the PWD. Additionally, the professional should take a detailed and thorough case history to identify any associated comorbidities so that the most effective and comprehensive interventions can be provided.
To increase the efficacy of identification, we recommend upon diabetes diagnosis that the PWD be referred to an audiologist for a baseline diagnostic audiological evaluation . Baseline evaluations can establish hearing status to improve ability to monitor change. An audiological evaluation should be performed at least every 2 years or sooner if an individual is experiencing any high-risk characteristics. PWD who are at high risk for hearing and balance problems may have one or more of the following characteristics and require closer monitoring:
Reduced speech understanding (particularly in noisy environments).
Tinnitus perception.
History of high levels of noise exposure.
History of ototoxic drug use.
Sensitivity to loud sounds.
Ear pain or drainage.
Dizziness complaints.
History of falls.
Concern for falling.
Low-risk individuals have none of these characteristics. Assessment of risk status identifies people who need more intensive care and evaluation.
Changes in the auditory system related to diabetes include sensory, neural, and vascular elements. 16 17 These changes can influence hearing along the range of human perception from regions associated with low-frequency and high-frequency hearing. Studies suggest that early indices of pathology may be related to subtle changes in cochlear, 46 auditory neural function, 11 and extended high-frequency hearing. 9 These factors should be considered when determining testing protocols. Based on the outcomes of the comprehensive audiological evaluation and case history, further testing including extended high-frequency threshold audiometry, immittance measures, OAEs, early- and late-auditory evoked potentials, tinnitus assessment, central auditory processing evaluation, and balance assessment may be recommended. The chosen test battery should be based on patient complaints and help determine the most appropriate intervention. Red flags, such as sudden hearing loss or evidence of disease, warrants a referral for medical evaluation.
Case Example
A patient with recent diagnosis of diabetes presents for baseline hearing assessment. The patient's complaints include aural fullness, tinnitus, and reduced speech understanding in noise. The patient denies any balance issues or experience of vertigo and denies any ear pain/drainage or history of ear infections. The patient's medications include metformin, furosemide, and sertraline. Results from the comprehensive audiological evaluation indicated a mild symmetrical high-frequency sensorineural hearing loss with excellent speech understanding in quiet. Based on these results, patient history, and patient's chief complaint, the provider may suggest further testing. For example, a recommendation may be made for OAEs to monitor subtle effects of medications on cochlear function. Speech-in-noise testing may be recommended to determine how noise affects speech understanding compared with understanding in quiet. If the patient's primary complaint is tinnitus, this may lead to recommendation for a tinnitus evaluation.
Continuum of care and counseling is also important. Strategies outlined in the primary prevention section should be reinforced during an evaluation or follow-up visit. PWD should be continually counseled on risk factors and preventative strategies for reducing risk for further hearing loss, including noise exposure, ototoxic medications, healthy lifestyle, and maintenance of ABCs.
A comprehensive audiological evaluation should be performed at least every 2 years or sooner if an individual is experiencing any high-risk characteristics. Based on patient history, complaints, and results of comprehensive audiological evaluation, further testing may be warranted. Sudden hearing loss or evidence of disease should result in a referral for a medical evaluation.
Early diagnosis and proper intervention can reduce risk for further acquired hearing loss and facilitate early treatment. The most common type of damage to the auditory system related to diabetes is sensory, neural, and vascular in nature, that is, sensorineural hearing loss, in contrast to increased risk for external and middle ear pathologies, where supporting data are limited. 26 Sensorineural hearing loss is commonly addressed by use of devices that amplify sound and/or aural rehabilitation strategies. PWD in early stages could have hearing loss in the range of mild to moderately severe in degree. 12 Considering their hearing demands, amplification and compensatory strategies can be advised. Surgical or pharmaceutical treatment of hearing loss currently has limited options in individuals with sensorineural hearing loss. Implantable devices such as a cochlear implant may be an option, but are reserved for patients with severe to profound hearing deficits and who derive limited benefit from hearing aids.
The maintenance of access to sound, communication, and quality of life with hearing loss is not different for the PWD as compared with a patient without diabetes and with hearing loss. Management options include communication strategies, aural rehabilitation, auditory training, assistive listening devices, hearing aids, and implantable devices. The recommended treatment is dependent on the type and degree of hearing loss and personal goals for functionality. There is currently no research on specific protocols for hearing loss management in PWD. Chartrand 47 provides an overview of some potential considerations including sensitivity to earmold materials, increased risk of ear canal infection/bleeding, higher odds of abnormal loudness perception, balance issues, and consideration of central auditory involvement. The provider should be cognizant of these concerns and exercise appropriate precautions.
PWD with hearing loss may choose to defer recommendations for amplification. The reasons may include lack of perceived deficit, stigma of hearing aids, or cost of devices. The patient should be counseled on communication strategies, alternative solutions for hearing loss (e.g., personal sound amplification products), other assistive listening devices, and relationship of untreated hearing with negative consequences.
The recommended treatment of hearing loss in PWD is always dependent on the impact of hearing loss impairment and personal goals for functionality.
Diabetes elevates risk for acquired forms of hearing loss. Here we have outlined primary, secondary, and tertiary prevention and management strategies for PWD. Health care providers including physicians, primary care providers, diabetes educators, and PPOD members should be cognizant of the relationship between diabetes and hearing loss. We recommend upon diagnosis that PWD undergo a baseline comprehensive audiological evaluation and follow-up at least every 2 years or sooner if high-risk characteristics are indicated. Furthermore, the PWD should be counseled on ways to reduce risk for acquired hearing loss including reducing noise exposure, adaption of healthy lifestyle, and maintaining ABCs. Management of hearing loss in PWD is comparable to traditional approaches for persons without diabetes; however, the provider should be aware of potential increased sensitivity to earmold materials and ear canal infection/bleeding.
Conflict of Interest The authors have nothing to disclose.
- 1. Akinpelu O V, Mujica-Mota M, Daniel S J. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope. 2014;124(03):767–776. doi: 10.1002/lary.24354. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Gupta S, Eavey R D, Wang M, Curhan S G, Curhan G C. Type 2 diabetes and the risk of incident hearing loss. Diabetologia. 2019;62(02):281–285. doi: 10.1007/s00125-018-4766-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Kim M B. Diabetes mellitus and the incidence of hearing loss: a cohort study. Int J Epidemiol. 2017;46(02):727. doi: 10.1093/ije/dyw342. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Cruickshanks K J, Nondahl D M, Dalton D S et al. Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. J Am Geriatr Soc. 2015;63(05):918–924. doi: 10.1111/jgs.13401. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Somogyi A, Rosta K, Vaszi T. [Hearing impairment and tinnitus in patients with type 2 diabetes] Orv Hetil. 2013;154(10):363–368. doi: 10.1556/OH.2013.29562. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Spankovich C, Le Prell C G, Lobarinas E, Hood L J. Noise history and auditory function in young adults with and without type 1 diabetes mellitus. Ear Hear. 2017;38(06):724–735. doi: 10.1097/AUD.0000000000000457. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Frisina S T, Mapes F, Kim S, Frisina D R, Frisina R D.Characterization of hearing loss in aged type II diabetics Hear Res 2006211(1-2):103–113. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Okhovat S A, Moaddab M H, Okhovat S H et al. Evaluation of hearing loss in juvenile insulin dependent patients with diabetes mellitus. J Res Med Sci. 2011;16(02):179–183. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Austin D F, Konrad-Martin D, Griest S, McMillan G P, McDermott D, Fausti S. Diabetes-related changes in hearing. Laryngoscope. 2009;119(09):1788–1796. doi: 10.1002/lary.20570. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Dalton D S, Cruickshanks K J, Klein R, Klein B E, Wiley T L. Association of NIDDM and hearing loss. Diabetes Care. 1998;21(09):1540–1544. doi: 10.2337/diacare.21.9.1540. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Konrad-Martin D, Austin D F, Griest S, McMillan G P, McDermott D, Fausti S. Diabetes-related changes in auditory brainstem responses. Laryngoscope. 2010;120(01):150–158. doi: 10.1002/lary.20636. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Bainbridge K E, Hoffman H J, Cowie C C. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med. 2008;149(01):1–10. doi: 10.7326/0003-4819-149-1-200807010-00231. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Bainbridge K E, Cheng Y J, Cowie C C. Potential mediators of diabetes-related hearing impairment in the U.S. population: National Health and Nutrition Examination Survey 1999-2004. Diabetes Care. 2010;33(04):811–816. doi: 10.2337/dc09-1193. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Bainbridge K E, Hoffman H J, Cowie C C. Risk factors for hearing impairment among U.S. adults with diabetes: National Health and Nutrition Examination Survey 1999-2004. Diabetes Care. 2011;34(07):1540–1545. doi: 10.2337/dc10-2161. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Bainbridge K E, Cowie C C, Gonzalez F, II et al. Risk factors for hearing impairment among adults with diabetes: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) J Clin Transl Endocrinol. 2016;6:15–22. doi: 10.1016/j.jcte.2016.09.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Fukushima H, Cureoglu S, Schachern P A, Paparella M M, Harada T, Oktay M F. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006;132(09):934–938. doi: 10.1001/archotol.132.9.934. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Fukushima H, Cureoglu S, Schachern P A et al. Cochlear changes in patients with type 1 diabetes mellitus. Otolaryngol Head Neck Surg. 2005;133(01):100–106. doi: 10.1016/j.otohns.2005.02.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Canlon B, Schacht J. Acoustic stimulation alters deoxyglucose uptake in the mouse cochlea and inferior colliculus. Hear Res. 1983;10(02):217–226. doi: 10.1016/0378-5955(83)90055-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11(12):3071–3109. doi: 10.1089/ars.2009.2484. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Lisowska G, Namysłowski G, Morawski K, Strojek K. Cochlear dysfunction and diabetic microangiopathy. Scand Audiol Suppl. 2001;(52):199–203. doi: 10.1080/010503901300007524. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. McQueen C T, Baxter A, Smith T L et al. Non-insulin-dependent diabetic microangiopathy in the inner ear. J Laryngol Otol. 1999;113(01):13–18. doi: 10.1017/s0022215100143051. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Smith T L, Raynor E, Prazma J, Buenting J E, Pillsbury H C.Insulin-dependent diabetic microangiopathy in the inner ear Laryngoscope 1995105(3, Pt 1):236–240. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Wackym P A, Linthicum F H., Jr Diabetes mellitus and hearing loss: clinical and histopathologic relationships. Am J Otol. 1986;7(03):176–182. [ PubMed ] [ Google Scholar ]
- 24. Anjaneyulu M, Berent-Spillson A, Russell J W. Metabotropic glutamate receptors (mGluRs) and diabetic neuropathy. Curr Drug Targets. 2008;9(01):85–93. doi: 10.2174/138945008783431772. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Liu F, Xia M, Xu A. Expression of VEGF, iNOS, and eNOS is increased in cochlea of diabetic rat. Acta Otolaryngol. 2008;128(11):1178–1186. doi: 10.1080/00016480801901774. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Chung J H, Lee S H, Park C W, Kim C, Park J K, Shin J H. Clinical significance of arterial stiffness in idiopathic sudden sensorineural hearing loss. Laryngoscope. 2016;126(08):1918–1922. doi: 10.1002/lary.25853. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Susmano A, Rosenbush S W. Hearing loss and ischemic heart disease. Am J Otol. 1988;9(05):403–408. [ PubMed ] [ Google Scholar ]
- 28. Bertrand R A, Huang Z. Association between audiometric patterns and probabilities of cardiovascular diseases. Laryngoscope Investig Otolaryngol. 2018;3(06):478–485. doi: 10.1002/lio2.206. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Wattamwar K, Qian Z J, Otter J et al. Association of cardiovascular comorbidities with hearing loss in the older old. JAMA Otolaryngol Head Neck Surg. 2018;144(07):623–629. doi: 10.1001/jamaoto.2018.0643. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Tay H L, Ray N, Ohri R, Frootko N J. Diabetes mellitus and hearing loss. Clin Otolaryngol Allied Sci. 1995;20(02):130–134. doi: 10.1111/j.1365-2273.1995.tb00029.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Bohne B A, Harding G W. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21(04):505–509. [ PubMed ] [ Google Scholar ]
- 32. Kukidome D, Nishikawa T, Sato M et al. Impaired balance is related to the progression of diabetic complications in both young and older adults. J Diabetes Complications. 2017;31(08):1275–1282. doi: 10.1016/j.jdiacomp.2017.05.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Jiam N T, Li C, Agrawal Y. Hearing loss and falls: a systematic review and meta-analysis. Laryngoscope. 2016;126(11):2587–2596. doi: 10.1002/lary.25927. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Palta P, Carlson M C, Crum R M et al. Diabetes and cognitive decline in older adults: the Ginkgo Evaluation of Memory Study. J Gerontol A Biol Sci Med Sci. 2017;73(01):123–130. doi: 10.1093/gerona/glx076. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Deal J A, Betz J, Yaffe K et al. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC study. J Gerontol A Biol Sci Med Sci. 2017;72(05):703–709. doi: 10.1093/gerona/glw069. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Konrad-Martin D, Reavis K M, Austin D et al. Hearing impairment in relation to severity of diabetes in a veteran cohort. Ear Hear. 2015;36(04):381–394. doi: 10.1097/AUD.0000000000000137. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Curhan S G, Wang M, Eavey R D, Stampfer M J, Curhan G C. Adherence to healthful dietary patterns is associated with lower risk of hearing loss in women. J Nutr. 2018;148(06):944–951. doi: 10.1093/jn/nxy058. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Spankovich C, Le Prell C G. Associations between dietary quality, noise, and hearing: data from the National Health and Nutrition Examination Survey, 1999-2002. Int J Audiol. 2014;53(11):796–809. doi: 10.3109/14992027.2014.921340. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Zhan W, Cruickshanks K J, Klein B Eet al. Modifiable determinants of hearing impairment in adults Prev Med 201153(4-5):338–342. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Loprinzi P D, Cardinal B J, Gilham B. Association between cardiorespiratory fitness and hearing sensitivity. Am J Audiol. 2012;21(01):33–40. doi: 10.1044/1059-0889(2011/11-0024). [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Loprinzi P D, Lee H, Gilham B, Cardinal B J. Association between accelerometer-assessed physical activity and tinnitus, NHANES 2005-2006. Res Q Exerc Sport. 2013;84(02):177–185. doi: 10.1080/02701367.2013.784840. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Loprinzi P D, Joyner C. Relationship between objectively measured physical activity, cardiovascular disease biomarkers, and hearing sensitivity using data from the National Health and Nutrition Examination Survey 2003-2006. Am J Audiol. 2017;26(02):163–169. doi: 10.1044/2017_AJA-16-0057. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Pirozzo S, Papinczak T, Glasziou P.Whispered voice test for screening for hearing impairment in adults and children: systematic review BMJ 2003327(7421):967. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Eekhof J A, de Bock G H, de Laat J A, Dap R, Schaapveld K, Springer M P. The whispered voice: the best test for screening for hearing impairment in general practice? Br J Gen Pract. 1996;46(409):473–474. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Strawbridge W J, Wallhagen M I. Simple tests compare well with a hand-held audiometer for hearing loss screening in primary care. J Am Geriatr Soc. 2017;65(10):2282–2284. doi: 10.1111/jgs.15044. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Spankovich C, Long G R, Hood L J. Early indices of reduced cochlear function in young adults with type-1 diabetes revealed by DPOAE fine structure. J Am Acad Audiol. 2019;30(06):459–471. doi: 10.3766/jaaa.17113. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Chartrand M S.Diabetes Mellitus and Hearing 2003. Available at: https://www.audiologyonline.com/articles/diabetes-mellitus-and-hearing-1120 . Accessed September 25, 2019 [ Google Scholar ]
- View on publisher site
- PDF (130.9 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Temporal Speech Parameters Indicate Early Cognitive Decline in Elderly Patients With Type 2 Diabetes Mellitus
Nóra imre , ma, réka balogh , ma, gábor gosztolya , phd, lászló tóth , phd, ildikó hoffmann , phd, tamás várkonyi , md, phd, csaba lengyel , md, phd, magdolna pákáski , md, phd, jános kálmán , md, phd, dsc.
- Author information
- Article notes
- Copyright and License information
Reprints: Nóra Imre, MA, Department of Psychiatry, Albert Szent-Györgyi Medical School, University of Szeged, Korányi fasor 8-10, Szeged H-6720, Hungary (e-mail: [email protected] ).
Corresponding author.
Received 2021 May 7; Accepted 2021 Dec 28; Issue date 2022 Apr-Jun.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc-nd/4.0/
Introduction:
The earliest signs of cognitive decline include deficits in temporal (time-based) speech characteristics. Type 2 diabetes mellitus (T2DM) patients are more prone to mild cognitive impairment (MCI). The aim of this study was to compare the temporal speech characteristics of elderly (above 50 y) T2DM patients with age-matched nondiabetic subjects.
Materials and Methods:
A total of 160 individuals were screened, 100 of whom were eligible (T2DM: n=51; nondiabetic: n=49). Participants were classified either as having healthy cognition (HC) or showing signs of MCI. Speech recordings were collected through a phone call. Based on automatic speech recognition, 15 temporal parameters were calculated.
The HC with T2DM group showed significantly shorter utterance length, higher duration rate of silent pause and total pause, and higher average duration of silent pause and total pause compared with the HC without T2DM group. Regarding the MCI participants, parameters were similar between the T2DM and the nondiabetic subgroups.
Conclusions:
Temporal speech characteristics of T2DM patients showed early signs of altered cognitive functioning, whereas neuropsychological tests did not detect deterioration. This method is useful for identifying the T2DM patients most at risk for manifest MCI, and could serve as a remote cognitive screening tool.
Key Words: mild cognitive impairment, type 2 diabetes mellitus, cognitive screening, neuropsychology, early detection, cognitive dysfunction, language functions, speech analysis, temporal speech characteristics, automatic speech recognition
Increasing evidence confirms the heightened risk of cognitive disorders in elderly patients living with type 2 diabetes mellitus (T2DM), compared with nondiabetic individuals. 1 , 2 T2DM not only doubles the odds of Alzheimers disease (AD) and vascular dementia (VD), 3 but also increases the incidence of mild cognitive impairment (MCI), the clinical condition between healthy aging and dementia. 4 MCI patients experience subtle cognitive symptoms (eg, deficits in language and executive functions, attention, or memory), which can cause problems with more complex activities of daily living but do not interfere with basic everyday functioning. 5 This association with cognitive decline poses a significant risk worldwide, as the global prevalence of T2DM is more than 9.3% of all adults today. 6 Although the exact pathophysiological pathways are under investigation, diabetes has been reported to accelerate the aging process of the brain through alterations in the metabolism of glucose, insulin, and amyloid, which can act as serious biological risk factors for dementia. 7 Cognition in T2DM was found to be impaired in several domains, like learning, verbal memory, attention, executive functions, processing and psychomotor speed, and language. 8
Decline in language functions have been found to be one of the earliest signs of cognitive deterioration. 9 Especially, the temporal (time-based) organization of speech reflects the functioning of several underlying cognitive processes, including the planning of speech production, the access to vocabulary, working memory, and, depending on the specific task, even episodic memory. 10 Studies using temporal analysis of speech found increased signs of disfluency (eg, word finding delays), or decreased speech rate in cognitively impaired individuals (eg, patients with AD or MCI). 11 – 13 Increased number/duration of pauses in speech is hypothesized to reflect the increased cognitive load required for maintaining one’s train of thought 14 and the general slowing down of word-retrieval. 9
Since temporal analyses of speech provide highly valuable information regarding cognitive processes, and there is a strong association between cognitive deficits and T2DM, it is of great significance to explore temporal speech characteristics among a high risk group, the elderly with T2DM. In the present study, an automated speech analysis method, the Speech-Gap Test (S-GAP Test) was applied on speech recordings of T2DM participants. This method, built on automatic speech recognition (ASR) techniques, was sensitive to distinguish between MCI patients and elderly individuals with healthy cognition (HC), both for Hungarian 15 – 19 and for English native speakers. 20
The objective of the present study was (1) to explore whether elderly HC individuals with and without T2DM differ in temporal speech characteristics, which may reflect subtle differences in cognition as well; and (2) to also understand how the same temporal speech characteristics compare between MCI patients with and without T2DM.
MATERIALS AND METHODS
Participants.
Based on the initial inclusion criteria, a total of 160 individuals were enrolled. After the exclusion process (Fig. 1 ), 100 of them were eligible for participation. Data collection took place at 2 departments of the Albert Szent-Györgyi Health Center, University of Szeged, Hungary: (1) T2DM patients were recruited at the Division of Diabetology of the Department of Internal Medicine, while (2) nondiabetic subjects were studied at the Memory Clinic of the Department of Psychiatry. The investigation took place within a 25-month time frame between 2018 and 2020.
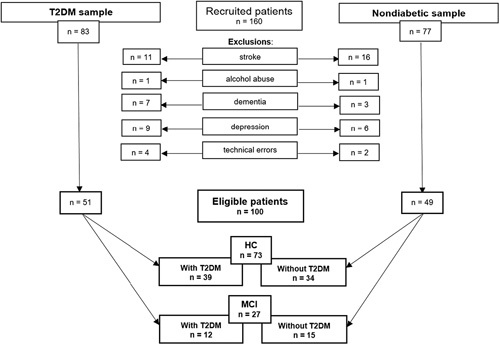
Demonstration of the inclusion/exclusion process, and the final sample sizes of the four study groups: HC with and without T2DM; MCI with and without T2DM. HC indicates healthy cognition; MCI, mild cognitive impairment; T2DM, type 2 diabetes mellitus.
Participation was voluntary after giving written informed consent. Participants did not receive financial compensation. The study was approved by the Regional Human Biomedical Research Ethics Committee of the University of Szeged, Hungary (231/2017-SZTE). The study was conducted in compliance with the principles of the Declaration of Helsinki.
All participants were evaluated by means of a neuropsychological battery (under Study protocol in detail). The battery included the Mini-Mental State Examination (MMSE), 21 which served as the measure of objective cognitive status. Based on the MMSE, participants were classified as either HC (30 to 28 points) or as having MCI (27 to 25 points). Finally, 4 groups emerged: HC with T2DM (n=39), HC without T2DM (n=34), MCI with T2DM (n=12), and MCI without T2DM (n=15).
Inclusion and Exclusion Process
Diabetes-related criteria.
In the T2DM sample, medical diagnosis of T2DM was the initial inclusion criterion. Diagnosis was based on current international guidelines of the American Diabetes Association. 22 Patients with type 1 diabetes mellitus, prediabetes, or chronic hyperglycemia of any other etiology were not enrolled. Average duration of diabetes was 11.4 years (SD=8.08); treatment was either oral medication (50.9%; n=26), insulin (25.5%; n=13), combined oral medication and insulin (17.6%; n=9), or only diet (5.9%; n=3).
Other Criteria
For all participants, initial inclusion criteria were a minimum age of 50 years, a minimum of 8 years of formal education, and Hungarian as native language. Exclusion criteria included the following: major hearing problems/deafness, acute depression, dementia, history of substance use disorder, head injuries, major neuropsychiatric disorders, previous computed tomography/magnetic resonance imaging showing evidence of significant abnormality suggesting another potential etiology for MCI (eg, prior macrohemorrhage/microhemorrhages, lacunar infarcts or single large infarct), evidence of cerebral contusion, encephalomalacia, aneurysm, vascular malformations or clinically significant space-occupying lesions. Finally, individuals whose speech could not be properly recorded due to technical errors were also excluded from further analysis (Fig. 1 ).
To check all inclusion and exclusion criteria, patient history was gathered from an initial interview and from available medical records. Furthermore, dementia and depression were screened on-site at the beginning of the protocol. The MMSE was used for dementia screening, and patients with a score under 25 were excluded. The presence/absence of acute depressive symptoms was evaluated by applying the 15-item Geriatric Depression Scale (GDS-15), 23 with a cut-off score of 6 above which individuals were not considered eligible.
Study Protocol
Neuropsychological tests.
Following a brief demographic and eligibility interview, a neuropsychological test sequence was administered, comprised of 8 instruments. These included 3 test batteries measuring current cognitive state: MMSE, Clock Drawing Test (CDT), 24 and Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) 25 ; 4 tests measuring working memory and executive functions: digit span test forward and backward, 26 nonword repetition test, 27 and listening span test 28 ; and one scale for measuring current depressive symptoms: GDS-15. The test order was fixed for all participants and had been assembled to ensure that tasks requiring the same cognitive function were separated (eg, working memory tasks did not directly follow each other).
Speech Task
A speech task was also administered to collect spontaneous (unplanned) speech samples for the temporal speech analysis. This task was chosen because it requires both working and episodic memory, allows remote and repeated testing, and was found to be sensitive in discriminating between MCI and controls. 19 In order to prevent fatigue, this speech task was administered approximately at the 15-minute mark of the 1-hour protocol. Speech was elicited in the following manner: the lead investigator (Investigator 1) told the participant that another researcher (Investigator 2), who was in a different room was to call them on a mobile phone and provide instructions for a new task. Following this cue, Investigator 2 called the participant and after a brief introduction, asked them to talk about their previous day. The standardized instruction was: “Please tell me about your previous day in as much detail as you can.” Following the instruction, both Investigator 1 (in the room) and Investigator 2 (on the phone) remained silent until the participant finished the task. The elicited monologue was recorded by a call recorder application installed on the mobile phone.
Speech Sample Preparation and Analysis
The obtained speech recordings were independently screened before analysis by 2 investigators: a linguist specialized in language pathologies (I.H.) screened the overall quality of the recording, while a researcher of computational speech analysis (G.G.) provided technical control. Those recordings that were not of suitable quality (n=4 in the T2DM, and n=2 in the nondiabetic groups) were excluded (Fig. 1 ). The remaining 100 recordings were converted into an uncompressed PCM mono, 16-bit wave format with a sampling rate of 8000 Hz, and were edited in the beginning and at the end so that only the participants’ speech remained; the opening/closing formulas and the instructions were removed.
After these preparations, ASR techniques were employed to identify pauses, both silent and filled, in each recording. Pauses were defined as the interruption of speech by either complete silence (silent pause) or by filler words like “um” or “er” (filled pause) lasting longer than 30 ms. The acoustic model was trained on a subset of the BEA audio corpus 29 that consisted of spontaneous speech, as this type of speech is expected to contain filled pauses (for the training of the ASR system, see Gosztolya et al 20 ). For training, the speech of 116 speakers was utilized, which amounted to ~44 hours of recordings. This ASR model performed phone-level recognition, with labeling of the input signal (including filled pauses, treated as a special “phoneme”) and the output of a phonetic segmentation. Based on the raw parameters from the ASR output, 15 temporal speech parameters were extracted using simple calculations established in previous works of our research group. 16 , 20 The calculations and definitions of the parameters are available as supplements (Supplemental Digital Content 1, http://links.lww.com/WAD/A379 ).
Statistical Analysis
Descriptive statistical data are expressed as means, medians, and SD for each group. The Shapiro-Wilk test demonstrated non-normality of data in most scale variables, thus the Mann-Whitney U test was employed to assess between-group differences on demographic data, neuropsychological test scores and temporal speech parameters. For categorical variables, Fisher exact test was applied. To further examine the abilities of each speech parameter in identifying T2DM patients, receiver operating characteristic (ROC) analysis was applied. Sensitivity and specificity (true positive and true negative rate) were calculated using threshold values that yielded the highest possible sensitivity (while keeping specificity above 50%). The level of significance was set at P <0.05 for all statistical tests. Analyses were performed using IBM SPSS 24.0 (SPSS Inc., Chicago, IL).
Demographic and Neuropsychological Characteristics
Demographic and neuropsychological test scores in the HC and MCI groups are presented in Table 1 , respectively. Within the HC sample, participants with T2DM and without T2DM did not differ statistically significantly in either of the demographic factors, or any of the neuropsychological tests. However, within the MCI sample, digit span (backwards) performance turned out to be significantly lower among the T2DM patients, compared with the nondiabetic participants.
Descriptive and Comparative Statistics of the Demographic Characteristics and Neuropsychological Test Scores in the HC With and Without T2DM, and the MCI With and Without T2DM Groups, Using the Mann-Whitney U Test or Fisher Exact Test (in Italics)
The P -values indicating statistically significant differences (at the P <0.05 level) are in bold.
ADAS-Cog indicates Alzheimer’s Disease Assessment Scale-Cognitive Subscale; CDT, Clock Drawing Test; GDS-15, 15-item Geriatric Depression Scale; HC, healthy cognition; M , mean; MCI, mild cognitive impairment; Mdn, median; MMSE, Mini-Mental State Examination; T2DM, type 2 diabetes mellitus.
Temporal Speech Parameters in the HC and MCI Groups According to Diabetic Status
Comparison between the T2DM and the nondiabetic groups was applied both within the HC and within the MCI samples. In the HC sample (Table 2 ), 5 of the 15 parameters differed significantly, as follows: the HC with T2DM group had shorter utterance length, higher duration rate of silent pause and total pause, and also higher average duration of silent pause and total pause, compared with the HC without T2DM group.
Descriptive and Comparative Statistics of the HC With and Without T2DM Groups Using the Mann-Whitney U Test
HC indicates healthy cognition; M, mean; Mdn, median; T2DM, type 2 diabetes mellitus.
A subsequent ROC analysis was executed in order to explore if HC with T2DM patients could be discriminated from HC without T2DM participants, based on their temporal speech parameters. The results showed that the same 5 parameters demonstrated significant classification potential, with utterance length having the highest area under the curve (AUC) (0.693) and the average duration of total pause yielding the highest sensitivity (79.5%). Sensitivity and specificity measures of temporal parameters were derived from ROC analysis; parameters with an AUC above 0.600 are shown in Table 4 .
Accuracy Measures of Temporal Parameters With AUC Above 0.600 in the HC and the MCI Samples, Respectively (Containing Both the “With T2DM” and “Without T2DM” Subgroups), Using Receiver Operating Characteristic (ROC) Analysis
The P -values indicating statistically significant classification abilities (at the P <0.05 level) are in bold.
AUC indicates area under the curve; CI, confidence interval; HC, healthy cognition; MCI, mild cognitive impairment; ROC, receiver operating characteristic; T2DM, type 2 diabetes mellitus.
However, regarding the MCI sample (Table 3 ), no statistically significant differences could be detected between the with and the without T2DM subgroups. This was further consolidated by the subsequent ROC analysis, which revealed that none of the 15 temporal parameters had statistically significant abilities to discriminate MCI with T2DM from MCI without T2DM participants. Nevertheless, parameters concerning filled pauses produced the highest AUCs. Sensitivity and specificity measures of temporal parameters were derived from ROC analysis; parameters with an AUC above 0.600 are shown in Table 4 .
Descriptive and Comparative Statistics of the MCI With and Without T2DM Groups Using the Mann-Whitney U Test
M indicates mean; MCI, mild cognitive impairment; Mdn, median; T2DM, type 2 diabetes mellitus.
Correlations of Temporal Speech Parameters With Age and Education
Regarding the relationship between age and the 15 temporal speech parameters across the 4 groups, correlation was statistically significant for articulation tempo (HC with T2DM: τ b =−0.221, P =0.050), for speech tempo (HC with T2DM: τ b =−0.229, P =0.042), and for silent pause frequency (MCI without T2DM: τ b =0.390, P =0.046). With regards to education, weak to moderate but statistically significant correlations were found with utterance length (HC without T2DM: τ b =0.269, P =0.035; MCI with T2DM: τ b =0.478, P =0.044), articulation tempo (MCI with T2DM: τ b =0.478, P =0.044), speech tempo (MCI with T2DM: τ b =0.546, P =0.021), filled pause occurrence rate (HC with T2DM: τ b =0.274, P =0.022), filled pause duration rate (HC with T2DM: τ b =0.268, P =0.025; MCI without T2DM: τ b =0.596, P =0.004), silent pause average duration (MCI with T2DM: τ b =−0.580, P =0.014), filled pause average duration (MCI without T2DM: τ b =−0.618, P =0.003), and total pause average duration (MCI with T2DM: τ b =−0.615, P =0.010). The comprehensive table containing all correlations is available as supplement (Supplemental Digital Content 1, http://links.lww.com/WAD/A379 ).
To the best of our knowledge, this was the first study that investigated the speech of T2DM patients with the purpose of detecting signs of subtle cognitive deficits that can manifest as changes in the temporal characteristics of speech. The major finding was that the speech of elderly HC individuals with T2DM compared significantly worse on several temporal characteristics to that of age-matched and education-matched HC individuals without T2DM.
Firstly, we intended to study the temporal speech characteristics of elderly T2DM patients who have been classified as HC based on traditional neuropsychological screening. Our results showed that their speech contains more signs of subtle, underlying cognitive deficits than that of the HC subjects without T2DM. Namely, 5 of 15 temporal speech parameters showed statistically significant differences between the diabetic and nondiabetic groups: HC with T2DM patients had shorter utterance length, higher duration rate of silent pause and total pause, and also higher average duration of silent pause and total pause compared to HC without T2DM participants. [Although it was not the focus of the present study, it is interesting to note that the temporal speech parameters that differentiated between the HC with/without T2DM groups also showed different mean/median values within the nondiabetic sample, between HC and MCI (Table 2 vs. Table 3 ). This further highlights that from the full set of 15 parameters these would have the most discriminative potential in future clinical applications.]
These differences are in agreement with the results of previous studies using the S-GAP Test and other speech analysis methods: in earlier works, more or longer pauses (signs of disfluency, word-finding difficulties and decreased lexical access) had been reported in the speech of patients with varying levels of cognitive impairment, for example, due to AD, 11 , 30 , 31 MCI, 12 , 13 or Parkinson disease. 32 , 33 These results, now complemented by the findings of the present study, confirm that pauses in speech provide a highly valuable source of information regarding language functions and thus cognitive state, especially in the introductory stages of neurocognitive disorders when other cognitive domains measured by traditional test batteries have not yet deteriorated in such a magnitude to be detected. In the case of T2DM patients, these subtle cognitive changes may be explained by diabetes-associated changes in the brain, such as impaired insulin signaling, neuronal insulin resistance, inflammation, mitochondrial dysfunction, vascular damage, or disturbances in synaptic plasticity, all of which can lead to an onset of cognitive decline. 7 , 34 , 35
Furthermore, we also compared the temporal speech characteristics of MCI patients with and without T2DM. No significant differences could be detected in any of the 15 analyzed temporal speech parameters, suggesting that these two groups performed similarly. A possible explanation for this could be that the pathophysiological processes in the brain are facilitated by T2DM and, as a consequence, cognitive abilities gradually deteriorate. According to current medical protocol, MCI diagnosis is only given when, besides fulfilling other criteria, cognitive symptoms reach a measurable level and can be confirmed by an objective evaluation tool. 36 , 37 However, it has been reported that the underlying cognitive deterioration is usually present for a longer period, more or less without clinical symptoms. 38 It could be argued that in the case of T2DM patients, the onset of the latent phase of transitioning from HC to MCI might take place earlier, and speech disfluencies might precede the more robust symptoms by a longer period of time than in the case of nondiabetic subjects. Our results also indicate that the temporal speech characteristics of T2DM and nondiabetic subjects tend to be similar when the cognitive deterioration reaches the level of MCI, which would suggest that once the transition to MCI has manifested, the presence of T2DM may not necessarily exacerbate the already deteriorated temporal speech symptoms. It would be of high clinical interest to further explore the effects of T2DM on cognition from a longitudinal viewpoint and to study whether temporal speech features differ in the next stage of cognitive decay, dementia with T2DM.
Regarding the relationship between demographics and temporal speech characteristics, age showed a statistically significant, weak correlation with 3 parameters: a negative correlation with articulation tempo and speech tempo, and a positive correlation with silent pause frequency. Education weakly to moderately correlated with 8 parameters: positively with utterance length, articulation tempo, speech tempo, filled pause occurrence rate, filled pause duration rate, and filled pause average duration; and negatively with silent pause average duration and total pause average duration. Careful examination of the positive and negative directions of the statistically significant correlations reveals that the increased presence of silent pauses (higher frequency or average length) was aligned with the demographic risk factors of cognitive decline (lower education, higher age 39 , 40 ). In contrast, the ability to produce more and faster speech (longer utterance length, higher articulation and speech tempo) was more associated with lower dementia-risk (such as higher education and lower age 39 ).
Limitations of the present study include the small number of MCI individuals which might reduce the statistical power of the comparisons, and therefore could contribute to the lack of between-group differences within the MCI sample. As this research was a pilot study for identifying speech parameters with the highest differentiating potential for future telemedicine-based assessments, multiple correction testing was not applied for the statistical comparisons. This needs to be taken into account when interpreting the results. On another note, even though the sampling rate used for speech recording (8000 Hz) might seem relatively low, the S-GAP Test was specifically intended to be applied in real-life settings, potentially in the form of a mobile application. A minimum sampling rate of 8000 Hz is available on most mobile phone devices, enabling wider adoption of the technology. Also, future works could also involve more diabetes-related medical characteristics, which could enable the creation of subgroups based on, for example, diabetes severity, glycemic control, or insulin levels.
The utilization of telemedicine in the management of diabetes is a dynamically emerging area, however, to this date no such technique has been used for the cognitive examination of diabetic patients. A subtle speech deficiency detected by the S-GAP Test could be an indication for a thorough medical and neuropsychological examination to search for possible underlying causes or for monitoring the patient more closely, for example, with frequent check-ups. Remote assessment is gaining increasing significance in light of the current COVID-19 pandemic, with every medical field facing restrictions of face-to-face appointments. The S-GAP Test is currently being developed in a mobile application format which could serve as a rapid, cost-effective, noninvasive, and no-contact form of cognitive screening for the elderly and, according to the present results, could be implemented for monitoring T2DM patients as well.
CONCLUSIONS
We explored the speech of T2DM patients, building on the shared pathophysiology of T2DM and neurocognitive disorders, as well as the strong association between cognitive deterioration and speech deficits. Even though T2DM patients classified as HC and matched nondiabetic subjects performed similarly on global cognitive and traditional neuropsychological tests, we demonstrated that the speech of T2DM patients contained an increased number and length of silent pauses compared to the nondiabetic group. Therefore, we would suggest that temporal analysis of speech offers a sensitive and ecologically valid tool for monitoring cognitive state in the early, introductory stages of cognitive impairments, and it could be useful for identifying the T2DM individuals who are more at risk of developing manifest MCI or later, dementia.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.alzheimerjournal.com .
Supported by the New National Excellence Program of the Ministry for Innovation and Technology from the Source of the National Research, Development and Innovation Fund, Hungary (N.I.: ÚNKP-21-4-I; G.G.: ÚNKP-21-5); the University of Szeged, Hungary (N.I., R.B.: EFOP-3.6.3-VEKOP-16-2017-00009); the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (G.G.: BO/00653/19); and the Hungarian Ministry for Innovation and Technology (G.G.: NKFIH-1279-2/2020, NKFIH-FK-124413).
The authors declare no conflicts of interest.
Contributor Information
Nóra Imre, Email: [email protected].
Réka Balogh, Email: [email protected].
Gábor Gosztolya, Email: [email protected].
László Tóth, Email: [email protected].
Ildikó Hoffmann, Email: [email protected].
Tamás Várkonyi, Email: [email protected].
Csaba Lengyel, Email: [email protected].
Magdolna Pákáski, Email: [email protected].
János Kálmán, Email: [email protected].
- 1. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Sadanand S, Balachandar R, Bharath S. Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2016;32:132–142. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer-disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Degen C, Toro P, Schönknecht P, et al. Diabetes mellitus type II and cognitive capacity in healthy aging, mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2016;240:42–46. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Jekel K, Damian M, Wattmo C, et al. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7:1–20. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Saeedi P, Petersohn I, Paraskevi S, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Szatloczki G, Hoffmann I, Vincze V, et al. Speaking in Alzheimer’s disease, is that an early sign? Importance of changes in language abilities in Alzheimer’s disease. Front Aging Neurosci. 2015;7:1–7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Mortensen L, Meyer AS, Humphreys GW. Age-related effects on speech production: a review. Lang Cogn Process. 2006;21:238–290. [ Google Scholar ]
- 11. Hoffmann I, Nemeth D, Dye CD, et al. Temporal features of spontaneous speech in Alzheimer’s disease. Int J Speech Lang Pathol. 2010;12:29–34. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Roark B, Mitchell M, Hosom JP, et al. Spoken language derived measures for detecting mild cognitive impairment. IEEE Trans Audio Speech Lang Processing. 2011;19:2081–2090. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Meilán JJG, Martínez-Sánchez F, Martínez-Nicolás I, et al. Changes in the rhythm of speech difference between people with nondegenerative mild cognitive impairment and with preclinical dementia. Behav Neurol. 2020;4683573:1–10. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. König A, Satt A, Sorin A, et al. Automatic speech analysis for the assessment of patients with predementia and Alzheimer’s disease. Alzheimers Dement. 2015;1:112–124. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Tóth L, Gosztolya G, Vincze V, et al. Automatic detection of mild cognitive impairment from spontaneous speech using ASR. Proceedings of Interspeech, Dresden, Germany. 2015: 2694–2698.
- 16. Tóth L, Hoffmann I, Gosztolya G, et al. A speech recognition-based solution for the automatic detection of mild cognitive impairment from spontaneous speech. Curr Alzheimer Res. 2018;15:130–138. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Gosztolya G, Tóth L, Grósz T, et al. Detecting mild cognitive impairment from spontaneous speech by correlation-based phonetic feature selection. Proceedings of Interspeech, San Francisco, USA. 2016:107–111.
- 18. Gosztolya G, Vincze V, Tóth L, et al. Identifying mild cognitive impairment and mild Alzheimer’s disease based on spontaneous speech using ASR and linguistic features. Comput Speech Lang. 2019;53:181–197. [ Google Scholar ]
- 19. Vincze V, Szatlóczki G, Tóth L, et al. Telltale silence: temporal speech parameters discriminate between prodromal dementia and mild Alzheimer’s disease. Clin Linguist Phon. 2021;35:727–742. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Gosztolya G, Balogh R, Imre N, et al. Cross-lingual detection of mild cognitive impairment based on temporal parameters of spontaneous speech. Comput Speech Lang. 2021;69:101215. [ Google Scholar ]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diab Care. 2014;37:S81–S90. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS)—recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [ Google Scholar ]
- 24. Shulman KI, Shedletsky R, Silver IL. The challenge of time: clock-drawing and cognitive function in the elderly. Int J Geriatric Psychiatry. 1986;1:135–140. [ Google Scholar ]
- 25. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Wechsler D. The Wechsler Adult Intelligence Scale—Revised. New York, NY: The Psychological Corporation; 1981. [ Google Scholar ]
- 27. Gathercole SE, Willis CS, Baddeley AD, et al. Gathercole SE, McCarthy RA. The children’s test of nonword repetition: a test of phonological working memory. Memory Tests and Techniques. Hove: Lawrence Erlbaum Associates; 1994:103–127. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verbal Learning Verbal Behav. 1980;19:450–466. [ Google Scholar ]
- 29. Neuberger T, Gyarmathy D, Gráczi TE, et al. Sojka P, Horák A, Kopeček I. Development of a large spontaneous speech database of agglutinative Hungarian language. Text, Speech and Dialogue. Brno, Czech Republic: Springer; 2014:424–431. [ Google Scholar ]
- 30. López-de-Ipiña K, Alonso J-B, Travieso CM, et al. On the selection of non-invasive methods based on speech analysis oriented to automatic Alzheimer disease diagnosis. Sensors (Basel). 2013;13:6730–6745. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Martínez-Sánchez F, Meilán JJG, García-Sevilla J. Oral reading fluency analysis in patients with Alzheimer disease and asymptomatic control subjects. Neurología. 2013;28:325–331. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Hlavnička J, Čmejla R, Tykalová T, et al. Automated analysis of connected speech reveals early biomarkers of Parkinson’s disease in patients with rapid eye movement sleep behaviour disorder. Sci Rep. 2017;7:1–12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Alvar AM, Lee J, Huber JE. Filled pauses as a special case of automatic speech behaviors and the effect of Parkinson’s disease. Am J Speech Lang Pathol. 2019;28(2S):835–843. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, et al. Pathophysiological mechanisms linking type 2 diabetes and dementia: review of evidence from clinical, translational and epidemiological research. Curr Diabetes Rev. 2019;15:456–470. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Stranahan AM, Arumugam TV, Cutler RG, et al. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Petersen RC, Smith GE, Warning SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Albert M, Yuxin Z, Moghekar A, et al. Predicting progression from normal cognition to mild cognitive impairment for individuals at 5 years. Brain. 2018;141:877–887. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Luck T, Luppa M, Briel S, et al. Incidence of mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord. 2010;29:164–175. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Patterson C, Feightner J, Garcia A, et al. General risk factors for dementia: a systematic evidence review. Alzheimers Dement. 2007;3:341–347. [ DOI ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (198.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Patient Care & Health Information
- Diseases & Conditions
- Type 2 diabetes
Type 2 diabetes is usually diagnosed using the glycated hemoglobin (A1C) test. This blood test indicates your average blood sugar level for the past two to three months. Results are interpreted as follows:
- Below 5.7% is normal.
- 5.7% to 6.4% is diagnosed as prediabetes.
- 6.5% or higher on two separate tests indicates diabetes.
If the A1C test isn't available, or if you have certain conditions that interfere with an A1C test, your health care provider may use the following tests to diagnose diabetes:
Random blood sugar test. Blood sugar values are expressed in milligrams of sugar per deciliter ( mg/dL ) or millimoles of sugar per liter ( mmol/L ) of blood. Regardless of when you last ate, a level of 200 mg/dL (11.1 mmol/L ) or higher suggests diabetes, especially if you also have symptoms of diabetes, such as frequent urination and extreme thirst.
Fasting blood sugar test. A blood sample is taken after you haven't eaten overnight. Results are interpreted as follows:
- Less than 100 mg/dL (5.6 mmol/L ) is considered healthy.
- 100 to 125 mg/dL (5.6 to 6.9 mmol/L ) is diagnosed as prediabetes.
- 126 mg/dL (7 mmol/L ) or higher on two separate tests is diagnosed as diabetes.
Oral glucose tolerance test. This test is less commonly used than the others, except during pregnancy. You'll need to not eat for a certain amount of time and then drink a sugary liquid at your health care provider's office. Blood sugar levels then are tested periodically for two hours. Results are interpreted as follows:
- Less than 140 mg/dL (7.8 mmol/L ) after two hours is considered healthy.
- 140 to 199 mg/dL (7.8 mmol/L and 11.0 mmol/L ) is diagnosed as prediabetes.
- 200 mg/dL (11.1 mmol/L ) or higher after two hours suggests diabetes.
Screening. The American Diabetes Association recommends routine screening with diagnostic tests for type 2 diabetes in all adults age 35 or older and in the following groups:
- People younger than 35 who are overweight or obese and have one or more risk factors associated with diabetes.
- Women who have had gestational diabetes.
- People who have been diagnosed with prediabetes.
- Children who are overweight or obese and who have a family history of type 2 diabetes or other risk factors.
After a diagnosis
If you're diagnosed with diabetes, your health care provider may do other tests to distinguish between type 1 and type 2 diabetes because the two conditions often require different treatments.
Your health care provider will test A1C levels at least two times a year and when there are any changes in treatment. Target A1C goals vary depending on age and other factors. For most people, the American Diabetes Association recommends an A1C level below 7%.
You also receive tests to screen for complications of diabetes and other medical conditions.

More Information
- Glucose tolerance test
Management of type 2 diabetes includes:
- Healthy eating.
- Regular exercise.
- Weight loss.
- Possibly, diabetes medication or insulin therapy.
- Blood sugar monitoring.
These steps make it more likely that blood sugar will stay in a healthy range. And they may help to delay or prevent complications.
Healthy eating
There's no specific diabetes diet. However, it's important to center your diet around:
- A regular schedule for meals and healthy snacks.
- Smaller portion sizes.
- More high-fiber foods, such as fruits, nonstarchy vegetables and whole grains.
- Fewer refined grains, starchy vegetables and sweets.
- Modest servings of low-fat dairy, low-fat meats and fish.
- Healthy cooking oils, such as olive oil or canola oil.
- Fewer calories.
Your health care provider may recommend seeing a registered dietitian, who can help you:
- Identify healthy food choices.
- Plan well-balanced, nutritional meals.
- Develop new habits and address barriers to changing habits.
- Monitor carbohydrate intake to keep your blood sugar levels more stable.
Physical activity
Exercise is important for losing weight or maintaining a healthy weight. It also helps with managing blood sugar. Talk to your health care provider before starting or changing your exercise program to ensure that activities are safe for you.
- Aerobic exercise. Choose an aerobic exercise that you enjoy, such as walking, swimming, biking or running. Adults should aim for 30 minutes or more of moderate aerobic exercise on most days of the week, or at least 150 minutes a week.
- Resistance exercise. Resistance exercise increases your strength, balance and ability to perform activities of daily living more easily. Resistance training includes weightlifting, yoga and calisthenics. Adults living with type 2 diabetes should aim for 2 to 3 sessions of resistance exercise each week.
- Limit inactivity. Breaking up long periods of inactivity, such as sitting at the computer, can help control blood sugar levels. Take a few minutes to stand, walk around or do some light activity every 30 minutes.
Weight loss
Weight loss results in better control of blood sugar levels, cholesterol, triglycerides and blood pressure. If you're overweight, you may begin to see improvements in these factors after losing as little as 5% of your body weight. However, the more weight you lose, the greater the benefit to your health. In some cases, losing up to 15% of body weight may be recommended.
Your health care provider or dietitian can help you set appropriate weight-loss goals and encourage lifestyle changes to help you achieve them.
Monitoring your blood sugar
Your health care provider will advise you on how often to check your blood sugar level to make sure you remain within your target range. You may, for example, need to check it once a day and before or after exercise. If you take insulin, you may need to check your blood sugar multiple times a day.
Monitoring is usually done with a small, at-home device called a blood glucose meter, which measures the amount of sugar in a drop of blood. Keep a record of your measurements to share with your health care team.
Continuous glucose monitoring is an electronic system that records glucose levels every few minutes from a sensor placed under the skin. Information can be transmitted to a mobile device such as a phone, and the system can send alerts when levels are too high or too low.
Diabetes medications
If you can't maintain your target blood sugar level with diet and exercise, your health care provider may prescribe diabetes medications that help lower glucose levels, or your provider may suggest insulin therapy. Medicines for type 2 diabetes include the following.
Metformin (Fortamet, Glumetza, others) is generally the first medicine prescribed for type 2 diabetes. It works mainly by lowering glucose production in the liver and improving the body's sensitivity to insulin so it uses insulin more effectively.
Some people experience B-12 deficiency and may need to take supplements. Other possible side effects, which may improve over time, include:
- Abdominal pain.
Sulfonylureas help the body secrete more insulin. Examples include glyburide (DiaBeta, Glynase), glipizide (Glucotrol XL) and glimepiride (Amaryl). Possible side effects include:
- Low blood sugar.
- Weight gain.
Glinides stimulate the pancreas to secrete more insulin. They're faster acting than sulfonylureas. But their effect in the body is shorter. Examples include repaglinide and nateglinide. Possible side effects include:
Thiazolidinediones make the body's tissues more sensitive to insulin. An example of this medicine is pioglitazone (Actos). Possible side effects include:
- Risk of congestive heart failure.
- Risk of bladder cancer (pioglitazone).
- Risk of bone fractures.
DPP-4 inhibitors help reduce blood sugar levels but tend to have a very modest effect. Examples include sitagliptin (Januvia), saxagliptin (Onglyza) and linagliptin (Tradjenta). Possible side effects include:
- Risk of pancreatitis.
- Joint pain.
GLP-1 receptor agonists are injectable medications that slow digestion and help lower blood sugar levels. Their use is often associated with weight loss, and some may reduce the risk of heart attack and stroke. Examples include exenatide (Byetta, Bydureon Bcise), liraglutide (Saxenda, Victoza) and semaglutide (Rybelsus, Ozempic, Wegovy). Possible side effects include:
SGLT2 inhibitors affect the blood-filtering functions in the kidneys by blocking the return of glucose to the bloodstream. As a result, glucose is removed in the urine. These medicines may reduce the risk of heart attack and stroke in people with a high risk of those conditions. Examples include canagliflozin (Invokana), dapagliflozin (Farxiga) and empagliflozin (Jardiance). Possible side effects include:
- Vaginal yeast infections.
- Urinary tract infections.
- Low blood pressure.
- High cholesterol.
- Risk of gangrene.
- Risk of bone fractures (canagliflozin).
- Risk of amputation (canagliflozin).
Other medicines your health care provider might prescribe in addition to diabetes medications include blood pressure and cholesterol-lowering medicines, as well as low-dose aspirin, to help prevent heart and blood vessel disease.
Insulin therapy
Some people who have type 2 diabetes need insulin therapy. In the past, insulin therapy was used as a last resort, but today it may be prescribed sooner if blood sugar targets aren't met with lifestyle changes and other medicines.
Different types of insulin vary on how quickly they begin to work and how long they have an effect. Long-acting insulin, for example, is designed to work overnight or throughout the day to keep blood sugar levels stable. Short-acting insulin generally is used at mealtime.
Your health care provider will determine what type of insulin is right for you and when you should take it. Your insulin type, dosage and schedule may change depending on how stable your blood sugar levels are. Most types of insulin are taken by injection.
Side effects of insulin include the risk of low blood sugar — a condition called hypoglycemia — diabetic ketoacidosis and high triglycerides.
Weight-loss surgery
Weight-loss surgery changes the shape and function of the digestive system. This surgery may help you lose weight and manage type 2 diabetes and other conditions related to obesity. There are several surgical procedures. All of them help people lose weight by limiting how much food they can eat. Some procedures also limit the amount of nutrients the body can absorb.
Weight-loss surgery is only one part of an overall treatment plan. Treatment also includes diet and nutritional supplement guidelines, exercise and mental health care.
Generally, weight-loss surgery may be an option for adults living with type 2 diabetes who have a body mass index (BMI) of 35 or higher. BMI is a formula that uses weight and height to estimate body fat. Depending on the severity of diabetes or the presence of other medical conditions, surgery may be an option for someone with a BMI lower than 35.
Weight-loss surgery requires a lifelong commitment to lifestyle changes. Long-term side effects may include nutritional deficiencies and osteoporosis.
People living with type 2 diabetes often need to change their treatment plan during pregnancy and follow a diet that controls carbohydrates. Many people need insulin therapy during pregnancy. They also may need to stop other treatments, such as blood pressure medicines.
There is an increased risk during pregnancy of developing a condition that affects the eyes called diabetic retinopathy. In some cases, this condition may get worse during pregnancy. If you are pregnant, visit an ophthalmologist during each trimester of your pregnancy and one year after you give birth. Or as often as your health care provider suggests.
Signs of trouble
Regularly monitoring your blood sugar levels is important to avoid severe complications. Also, be aware of symptoms that may suggest irregular blood sugar levels and the need for immediate care:
High blood sugar. This condition also is called hyperglycemia. Eating certain foods or too much food, being sick, or not taking medications at the right time can cause high blood sugar. Symptoms include:
- Frequent urination.
- Increased thirst.
- Blurred vision.
Hyperglycemic hyperosmolar nonketotic syndrome (HHNS). This life-threatening condition includes a blood sugar reading higher than 600 mg/dL (33.3 mmol/L ). HHNS may be more likely if you have an infection, are not taking medicines as prescribed, or take certain steroids or drugs that cause frequent urination. Symptoms include:
- Extreme thirst.
- Drowsiness.
- Dark urine.
Diabetic ketoacidosis. Diabetic ketoacidosis occurs when a lack of insulin results in the body breaking down fat for fuel rather than sugar. This results in a buildup of acids called ketones in the bloodstream. Triggers of diabetic ketoacidosis include certain illnesses, pregnancy, trauma and medicines — including the diabetes medicines called SGLT2 inhibitors.
The toxicity of the acids made by diabetic ketoacidosis can be life-threatening. In addition to the symptoms of hyperglycemia, such as frequent urination and increased thirst, ketoacidosis may cause:
- Shortness of breath.
- Fruity-smelling breath.
Low blood sugar. If your blood sugar level drops below your target range, it's known as low blood sugar. This condition also is called hypoglycemia. Your blood sugar level can drop for many reasons, including skipping a meal, unintentionally taking more medication than usual or being more physically active than usual. Symptoms include:
- Irritability.
- Heart palpitations.
- Slurred speech.
If you have symptoms of low blood sugar, drink or eat something that will quickly raise your blood sugar level. Examples include fruit juice, glucose tablets, hard candy or another source of sugar. Retest your blood in 15 minutes. If levels are not at your target, eat or drink another source of sugar. Eat a meal after your blood sugar level returns to normal.
If you lose consciousness, you need to be given an emergency injection of glucagon, a hormone that stimulates the release of sugar into the blood.
- Medications for type 2 diabetes
- GLP-1 agonists: Diabetes drugs and weight loss
- Bariatric surgery
- Endoscopic sleeve gastroplasty
- Gastric bypass (Roux-en-Y)
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
Careful management of type 2 diabetes can reduce the risk of serious — even life-threatening — complications. Consider these tips:
- Commit to managing your diabetes. Learn all you can about type 2 diabetes. Make healthy eating and physical activity part of your daily routine.
- Work with your team. Establish a relationship with a certified diabetes education specialist, and ask your diabetes treatment team for help when you need it.
- Identify yourself. Wear a necklace or bracelet that says you are living with diabetes, especially if you take insulin or other blood sugar-lowering medicine.
- Schedule a yearly physical exam and regular eye exams. Your diabetes checkups aren't meant to replace regular physicals or routine eye exams.
- Keep your vaccinations up to date. High blood sugar can weaken your immune system. Get a flu shot every year. Your health care provider also may recommend the pneumonia vaccine. The Centers for Disease Control and Prevention (CDC) also recommends the hepatitis B vaccination if you haven't previously received this vaccine and you're 19 to 59 years old. Talk to your health care provider about other vaccinations you may need.
- Take care of your teeth. Diabetes may leave you prone to more-serious gum infections. Brush and floss your teeth regularly and schedule recommended dental exams. Contact your dentist right away if your gums bleed or look red or swollen.
- Pay attention to your feet. Wash your feet daily in lukewarm water, dry them gently, especially between the toes, and moisturize them with lotion. Check your feet every day for blisters, cuts, sores, redness and swelling. Contact your health care provider if you have a sore or other foot problem that isn't healing.
- Keep your blood pressure and cholesterol under control. Eating healthy foods and exercising regularly can go a long way toward controlling high blood pressure and cholesterol. Take medication as prescribed.
- If you smoke or use other types of tobacco, ask your health care provider to help you quit. Smoking increases your risk of diabetes complications. Talk to your health care provider about ways to stop using tobacco.
- Use alcohol sparingly. Depending on the type of drink, alcohol may lower or raise blood sugar levels. If you choose to drink alcohol, only do so with a meal. The recommendation is no more than one drink daily for women and no more than two drinks daily for men. Check your blood sugar frequently after drinking alcohol.
- Make healthy sleep a priority. Many people with type 2 diabetes have sleep problems. And not getting enough sleep may make it harder to keep blood sugar levels in a healthy range. If you have trouble sleeping, talk to your health care provider about treatment options.
- Caffeine: Does it affect blood sugar?
Alternative medicine
Many alternative medicine treatments claim to help people living with diabetes. According to the National Center for Complementary and Integrative Health, studies haven't provided enough evidence to recommend any alternative therapies for blood sugar management. Research has shown the following results about popular supplements for type 2 diabetes:
- Chromium supplements have been shown to have few or no benefits. Large doses can result in kidney damage, muscle problems and skin reactions.
- Magnesium supplements have shown benefits for blood sugar control in some but not all studies. Side effects include diarrhea and cramping. Very large doses — more than 5,000 mg a day — can be fatal.
- Cinnamon, in some studies, has lowered fasting glucose levels but not A1C levels. Therefore, there's no evidence of overall improved glucose management.
Talk to your health care provider before starting a dietary supplement or natural remedy. Do not replace your prescribed diabetes medicines with alternative medicines.
Coping and support
Type 2 diabetes is a serious disease, and following your diabetes treatment plan takes commitment. To effectively manage diabetes, you may need a good support network.
Anxiety and depression are common in people living with diabetes. Talking to a counselor or therapist may help you cope with the lifestyle changes and stress that come with a type 2 diabetes diagnosis.
Support groups can be good sources of diabetes education, emotional support and helpful information, such as how to find local resources or where to find carbohydrate counts for a favorite restaurant. If you're interested, your health care provider may be able to recommend a group in your area.
You can visit the American Diabetes Association website to check out local activities and support groups for people living with type 2 diabetes. The American Diabetes Association also offers online information and online forums where you can chat with others who are living with diabetes. You also can call the organization at 800-DIABETES ( 800-342-2383 ).
Preparing for your appointment
At your annual wellness visit, your health care provider can screen for diabetes and monitor and treat conditions that increase your risk of diabetes, such as high blood pressure, high cholesterol or a high BMI .
If you are seeing your health care provider because of symptoms that may be related to diabetes, you can prepare for your appointment by being ready to answer the following questions:
- When did your symptoms begin?
- Does anything improve the symptoms or worsen the symptoms?
- What medicines do you take regularly, including dietary supplements and herbal remedies?
- What are your typical daily meals? Do you eat between meals or before bedtime?
- How much alcohol do you drink?
- How much daily exercise do you get?
- Is there a history of diabetes in your family?
If you are diagnosed with diabetes, your health care provider may begin a treatment plan. Or you may be referred to a doctor who specializes in hormonal disorders, called an endocrinologist. Your care team also may include the following specialists:
- Certified diabetes education specialist.
- Foot doctor, also called a podiatrist.
- Doctor who specializes in eye care, called an ophthalmologist.
Talk to your health care provider about referrals to other specialists who may be providing care.
Questions for ongoing appointments
Before any appointment with a member of your treatment team, make sure you know whether there are any restrictions, such as not eating or drinking before taking a test. Questions that you should regularly talk about with your health care provider or other members of the team include:
- How often do I need to monitor my blood sugar, and what is my target range?
- What changes in my diet would help me better manage my blood sugar?
- What is the right dosage for prescribed medications?
- When do I take the medications? Do I take them with food?
- How does management of diabetes affect treatment for other conditions? How can I better coordinate treatments or care?
- When do I need to make a follow-up appointment?
- Under what conditions should I call you or seek emergency care?
- Are there brochures or online sources you recommend?
- Are there resources available if I'm having trouble paying for diabetes supplies?
What to expect from your doctor
Your health care provider is likely to ask you questions at your appointments. Those questions may include:
- Do you understand your treatment plan and feel confident you can follow it?
- How are you coping with diabetes?
- Have you had any low blood sugar?
- Do you know what to do if your blood sugar is too low or too high?
- What's a typical day's diet like?
- Are you exercising? If so, what type of exercise? How often?
- Do you sit for long periods of time?
- What challenges are you experiencing in managing your diabetes?
- Professional Practice Committee: Standards of Medical Care in Diabetes — 2020. Diabetes Care. 2020; doi:10.2337/dc20-Sppc.
- Diabetes mellitus. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetes-mellitus-dm. Accessed Dec. 7, 2020.
- Melmed S, et al. Williams Textbook of Endocrinology. 14th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 3, 2020.
- Diabetes overview. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/all-content. Accessed Dec. 4, 2020.
- AskMayoExpert. Type 2 diabetes. Mayo Clinic; 2018.
- Feldman M, et al., eds. Surgical and endoscopic treatment of obesity. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 20, 2020.
- Hypersmolar hyperglycemic state (HHS). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hyperosmolar-hyperglycemic-state-hhs. Accessed Dec. 11, 2020.
- Diabetic ketoacidosis (DKA). Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetic-ketoacidosis-dka. Accessed Dec. 11, 2020.
- Hypoglycemia. Merck Manual Professional Version. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/hypoglycemia. Accessed Dec. 11, 2020.
- 6 things to know about diabetes and dietary supplements. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/tips/things-to-know-about-type-diabetes-and-dietary-supplements. Accessed Dec. 11, 2020.
- Type 2 diabetes and dietary supplements: What the science says. National Center for Complementary and Integrative Health. https://www.nccih.nih.gov/health/providers/digest/type-2-diabetes-and-dietary-supplements-science. Accessed Dec. 11, 2020.
- Preventing diabetes problems. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/all-content. Accessed Dec. 3, 2020.
- Schillie S, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and Reports. 2018; doi:10.15585/mmwr.rr6701a1.
- Diabetes prevention: 5 tips for taking control
- Hyperinsulinemia: Is it diabetes?
Associated Procedures
News from mayo clinic.
- Mayo Clinic, Carnegie Mellon researchers secure ARPA-H award for multiyear implantable device research for diabetes and obesity Oct. 02, 2024, 03:00 p.m. CDT
- Mayo study uses electronic health record data to assess metformin failure risk, optimize care Feb. 10, 2023, 02:30 p.m. CDT
- Mayo Clinic Minute: Strategies to break the heart disease and diabetes link Nov. 28, 2022, 05:15 p.m. CDT
Products & Services
- A Book: The Essential Diabetes Book
- A Book: The Mayo Clinic Diabetes Diet
- Assortment of Health Products from Mayo Clinic Store
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- NEW: Listen to Health Matters Podcast - Mayo Clinic Press NEW: Listen to Health Matters Podcast
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Wednesday, October 9, 2024
NIH launches large study to tackle type 2 diabetes in young people
Effort to identify risk factors for youth-onset type 2 diabetes to improve prevention and treatment.
The National Institutes of Health has launched a nationwide consortium to address the dramatic rise in youth diagnosed with type 2 diabetes over the past two decades, a trend that is expected to continue. The effort aims to advance understanding of the biologic, social, and environmental drivers of youth-onset type 2 diabetes, with the goals of determining which children are at highest risk for developing the disease and how to better prevent, screen for, and manage type 2 diabetes in young people.
“Our children who are overweight or have obesity are at risk, but we don't know how best to identify the children who will progress to type 2 diabetes,” said Rose Gubitosi-Klug, M.D., Ph.D., study lead, and chief of pediatric endocrinology at Case Western Reserve University/Rainbow Babies and Children’s Hospital, Cleveland. “This study will bring us closer to our goal of prevention of type 2 diabetes in future generations of youth.”
The observational study is funded by the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and builds on previous NIDDK-funded research indicating that youth-onset type 2 diabetes is more challenging to treat and progresses more aggressively compared to adult-onset type 2 diabetes. In youth with type 2 diabetes, good blood glucose control is harder to achieve, and the ability of the pancreas to secrete insulin declines much more rapidly. Many young people with type 2 diabetes also don’t respond well to metformin, the drug most commonly used as the first-line treatment for diabetes in adults. In addition, youth-onset type 2 diabetes is associated with earlier development of diabetes-related complications, such as damage to the eyes, kidneys, and nerves.
“These factors all create a picture of a disease that is much more aggressive in youth than in adults, but we don’t understand what drives these differences,” said Barbara Linder, M.D., Ph.D., NIDDK program director who is overseeing the study. “Consequently, young people are developing devastating complications of the disease during what should be the most productive years of their life.”
The study will aim to identify unique drivers of youth-onset type 2 diabetes distinguishing it from the disease in adults, which will help clinicians better understand which children will develop the disease and guide more effective, targeted prevention and intervention strategies. Study sites across the country will recruit 3,600 participants, ages 9 to 14, who are considered at risk for developing type 2 diabetes. They must have started puberty, have overweight or obesity, and have high-normal to above-normal hemoglobin A1c (HbA1c) levels but not high enough for a diagnosis of diabetes. The participants will reflect the U.S. population of youth with type 2 diabetes, including people from diverse racial and ethnic, socioeconomically disadvantaged, and underserved rural populations.
The research team is also seeking extensive input from youth, young adults, and parents with lived experience of type 2 diabetes on both study design and conduct, including how to best recruit and retain participants, how frequently participants should be seen during the study, what questionnaires should be used to collect data, and more.
In addition to looking at biological factors, the study team will gather comprehensive data from participants and their families to understand what social and environmental factors may be adversely contributing to health disparities and poor outcomes among youth with type 2 diabetes. Research has suggested that these social determinants of health—the conditions in which people are born, grow, work, live, and age—have a powerful influence on shaping health outcomes. For example, people without access to healthy food and safe places to engage in physical activity may be more likely to develop obesity, which is associated with type 2 diabetes.
“Most children we currently consider ‘at-risk’ for developing type 2 diabetes will not actually do so, so we need to better understand what factors define who is at risk and would benefit from targeted prevention strategies,” said Dr. Linder. “These efforts are critical to lessen the immense burden, not just on young people and their families, but also the U.S. healthcare system, arising from the growing numbers of youth living with this disease and its debilitating complications.”
For more information about the study, known as DISCOVERY of Risk Factors for Type 2 Diabetes in Youth, please visit discovery.bsc.gwu.edu .
Funding: DISCOVERY is funded through NIH grants DK134971, DK134984, DK134975, DK134996, DK134958, DK134967, DK135002, DK134982, DK135007, DK134988, DK134978, DK134981, DK135012, DK135015, DK134976, and DK134966.
The NIDDK, a component of the NIH, conducts and supports research on diabetes and other endocrine and metabolic diseases; digestive diseases, nutrition and obesity; and kidney, urologic and hematologic diseases. Spanning the full spectrum of medicine and afflicting people of all ages and ethnic groups, these diseases encompass some of the most common, severe, and disabling conditions affecting Americans. For more information about the NIDDK and its programs, see https://www.niddk.nih.gov .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
Talk to us about diabetes
0345 123 2399
customer support
Sugar and diabetes
You don’t need to cut out sugar from your diet if you have diabetes. and while we don’t know exactly what causes type 1 diabetes , but it isn’t linked to lifestyle, and so sugar doesn’t directly cause the condition. .
The question of whether sugar directly causes type 2 diabetes is a bit complicated.
Because diabetes is a condition where blood sugar levels are too high, it’s all too easy to think eating too much sugar is the cause. But what’s the truth about sugar and how does it affect diabetes?
In this article we’ll explain whether sugar causes diabetes , how to cut down on sugar and how to read food labels to make informed decisions about your diet.
Where sugar is found in your diet
Sugar is found naturally in fruit, vegetables (fructose) and dairy foods (lactose). It’s also added to food and drink by food manufacturers, or by ourselves at home. These types of added sugars are called ‘free sugars’ and they are also present in pure fruit juices, smoothies, syrups and honey. The debate about sugar and health is mainly around free sugars.
This includes:
- table sugar that we add to our hot drinks or breakfast cereal
- caster sugar, used in baking
- sugars hidden in sauces, ready meals, cakes and drinks.
- honey and syrups, like golden syrup or agave syrup
- pure fruit juice
- smoothies.
Does sugar cause diabetes?
There are two main types of diabetes – type 1 and type 2 diabetes.
We know that sugar does not cause type 1 diabetes, nor is it caused by anything else in your lifestyle. In type 1 diabetes, the insulin producing cells in your pancreas are destroyed by your immune system.
With type 2 diabetes, the answer is a little more complex. Though we know sugar doesn’t directly cause type 2 diabetes, you are more likely to get it if you are overweight. You gain weight when you take in more calories than your body needs, and sugary foods and drinks contain a lot of calories.
So you can see if too much sugar is making you put on weight, then you are increasing your risk of getting type 2 diabetes. But type 2 diabetes is complex, and sugar is unlikely to be the only reason the condition develops.
We also know that sugar sweetened drinks, like canned soft drinks, are associated with an increased risk of type 2 diabetes, and this is not necessarily linked to their effect on body weight.
Find out your risk of type 2 diabetes .
Sugar and diabetes and your diet
We all enjoy eating sugary foods occasionally, and there’s no problem including them as a treat occasionally as part of a healthy, balanced diet . And, for some people with diabetes, sugary drinks or glucose tablets are essential to treat a hypo , when your blood glucose levels get too low.
However, we are eating too much free sugar and harming our health as a result. Being overweight can make it difficult to manage your diabetes and increase your risk of getting serious health problems such as heart disease and stroke in the future. Too much sugar is bad for your teeth too.
The maximum recommended daily amount of sugar is 30g for adults – which works out at just seven teaspoons a day. Given that a tablespoon of ketchup contains around one teaspoon of sugar, a chocolate biscuit has up to two, and a small serving of baked beans almost three, you can see how quickly the teaspoons tot up.
How to cut down on sugar
You don’t have to cut sugar out of your diet completely. Sugar is found naturally in fruit, vegetables and dairy foods, and most of us in the UK are not getting the recommended five fruit and veg a day so it’s important we don’t cut these out as they are so good for you.
It’s better to eat whole fruit and vegetables rather than having juices or smoothies, as even the pure fruit juices contribute to free sugar intake. If you do have juice, keep to just one small glass – 150ml – a day.
It’s the free sugar that we all need to cut down on. And it’s not just the obviously sweet things like biscuits and chocolate. It’s the hidden sugar lurking in many foods, such as baked beans, pasta sauces, tomato ketchup, yogurts and ready meals. Some drinks are packed with sugar, too.
Simple changes can dramatically reduce the amount of free sugar in your diet. This could include:
- Instead of chocolate bars, sweets, cakes and biscuits, choose healthier snacks such as unsweetened yogurts, unsalted nuts, seeds, fruits and vegetables. For example, try natural yogurt mixed in with chopped fruit or a small handful of nuts.
- Experiment with reducing the sugar you use in recipes – most recipes will work just as well.
- Try artificial sweetener in place of sugar.
- If you normally have sugary drinks, choose diet fizzy drinks and no added sugar squashes instead. Or go for water with natural flavourings, like mint or sliced lemon. Sugary drinks are best used as a treatment for hypos .
- Try to cook from scratch where possible. That way you can be sure of what's in your food. Check out our tasty, easy-to-follow and simple recipes .
- Keep an eye on reduced-fat foods. Many actually contain more sugar as food manufactures add sugar to compensate for the altered taste and texture caused by the fat being removed. Look at the whole food label to be sure.
"Low-fat foods, such as yogurts, can be higher in sugar, so always check labels for ingredients.” Margaret, 73, who has type 2 diabetes
Reading food labels
Food labels are the best way to work out how much sugar is in what you're eating. The figures for sugar are for the total sugar in that food item, and don’t tell you how much of the sugar comes from natural sources, such as in fruit, and how much comes from free sugar.
Some foods and drink don’t have the word ‘sugar’ in the ingredients list, but still have sugar added. Honey, sucrose, glucose, glucose syrup, dextrose, fructose, hydrolysed starch, corn and maize syrup are all free sugars. If you see any of these words on the ingredients list, you know sugar has been added.
To see whether a product is high in free sugar look at the ingredients list, which always starts with the biggest ingredient first. So if sugar or syrup is listed in the first few ingredients, the product you’re buying will contain a high proportion of sugar.
Read more about understanding food labels .
Back to the top
Share this Page
© The British Diabetic Association operating as Diabetes UK, a charity registered in England and Wales (no. 215199) and in Scotland (no. SC039136). A company limited by guarantee registered in England and Wales with (no.00339181) and registered office at Wells Lawrence House, 126 Back Church Lane London E1 1FH

- health-fitness
- health-news
World Diabetes Day 2024: Early and surprising signs of the silent killer
Silent and surprising signs of diabetes


IMAGES
VIDEO
COMMENTS
However, a new study suggests that type 2 diabetes could also be detected in a person's voice. Researchers from Klick Labs have created an artificial intelligence (AI) tool that can determine if a person has type 2 diabetes using just six to 10 seconds of their voice, combined with basic health information like age, sex, height, and weight.
Introduction. Diabetes mellitus is known to affect the neurological, vascular and muscular systems. The American Diabetes Association has described the major symptoms of diabetes as follows: polydipsia, polyphagia, polyuria, extreme fatigue, blurred vision, slow healing sores, weight loss, tingling, pain or numbness in hands. 1 Further, a high prevalence of gastrointestinal symptoms has been ...
The shifts may be far more subtle (or completely nonexistent) in people with recently developed type 2 diabetes, which might reduce the voice-recognition technique's efficacy as a tool for screening or diagnosis. Kaufman told Diabetes Daily that "we have also considered this question and, to address it, we are looking to perform the same ...
An international study, Colive Voice, presented at the European Association for the Study of Diabetes (EASD) 2024 conference, shows that patients with type 2 diabetes (T2D) have different voice ...
Abstract. Recent research describes the effect of Type 2 diabetes (T2D) on voice, suggesting that it can be diagnosed based on vocal clues. Although these studies have similar experimental designs with respect to the voice data and the analysis methods, the conclusions regarding the voice changes differ substantially and are at times contradictory.
Type 2 diabetes mellitus (DM2) is associated with impaired hearing. However, the evidence is less clear if DM2 can lead to difficulty understanding speech in complex acoustic environments, independently of age and hearing loss effects.
The authors aimed to answer the following question: does type 2 diabetes have an effect on voice? Methods: The systematic review included search terms such as "speech, voice, larynx, glucose, diabetes, and hyperglycemia." The search strategy yielded 221 articles, only five of which satisfied the inclusion criteria.
Diabetes Australia believes that optimal communication increases motivation, health and the well-being of people with diabetes. Furthermore, careless or negative language can be demotivating, is often inaccurate, and can be harmful. In 2017, The Use of Language in Diabetes Care and Educatio n was published.
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by impaired insulin action and elevated blood glucose levels. ... fixed sentence despite the reported success in determining glucose-related voice changes from spoken sentences and free speech. 4 This manuscript aims to address the following points to identify vocal ...
The type of hearing loss most commonly seen in the population with type 2 diabetes is presbycusis—bilateral high frequency and sensorineural hearing loss. The loss is permanent and is usually progressive. ... ideal initial management team would consist of a primary care physician, endocrinologist, cardiologist, audiologist, speech-language ...
Speech-in-noise testing may be recommended to determine how noise affects speech understanding compared with understanding in quiet. If the patient's primary complaint is tinnitus, this may lead to recommendation for a tinnitus evaluation. ... Somogyi A, Rosta K, Vaszi T. [Hearing impairment and tinnitus in patients with type 2 diabetes] Orv ...
The present study aimed to examine the prevalence of hearing loss in patients with type 2 diabetes. A total of 94 patients with type 2 diabetes, aged from 40 to 80 years, were selected randomly in the present descriptive cross-sectional study for pure tone audiometry (PTA), speech discrimination score (SDS), and speech reception threshold (SRT ...
Summary. Recent research describes the effect of Type 2 diabetes (T2D) on voice, suggesting that it can be diagnosed based on vocal clues. Although these studies have similar experimental designs with respect to the voice data and the analysis methods, the conclusions regarding the voice changes differ substantially and are at times contradictory.
Purpose: Type 2 diabetes mellitus (DM2) is associated with impaired hearing. However, the evidence is less clear if DM2 can lead to difficulty understanding speech in complex acoustic environments, independently of age and hearing loss effects. The purpose of this study was to estimate the magnitude of DM2-related effects on speech understanding
The earliest signs of cognitive decline include deficits in temporal (time-based) speech characteristics. Type 2 diabetes mellitus (T2DM) patients are more prone to mild cognitive impairment (MCI). The aim of this study was to compare the temporal speech characteristics of elderly (above 50 y) T2DM patients with age-matched nondiabetic subjects.
Causes. Type 2 diabetes is mainly the result of two problems: Cells in muscle, fat and the liver become resistant to insulin As a result, the cells don't take in enough sugar. The pancreas can't make enough insulin to keep blood sugar levels within a healthy range. Exactly why this happens is not known.
Slurred speech. Drowsiness. Confusion. ... Type 2 diabetes is a serious disease, and following your diabetes treatment plan takes commitment. To effectively manage diabetes, you may need a good support network. Anxiety and depression are common in people living with diabetes. Talking to a counselor or therapist may help you cope with the ...
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by impaired insulin action and elevated blood glucose levels. ... fixed sentence despite the reported success in determining glucose-related voice changes from spoken sentences and free speech. 4 This manuscript aims to address the following points to identify vocal ...
In youth with type 2 diabetes, good blood glucose control is harder to achieve, and the ability of the pancreas to secrete insulin declines much more rapidly. Many young people with type 2 diabetes also don't respond well to metformin, the drug most commonly used as the first-line treatment for diabetes in adults. In addition, youth-onset ...
There are two main types of diabetes - type 1 and type 2 diabetes. We know that sugar does not cause type 1 diabetes, nor is it caused by anything else in your lifestyle. In type 1 diabetes, the insulin producing cells in your pancreas are destroyed by your immune system. With type 2 diabetes, the answer is a little more complex.
For Type 2 diabetes, while weight gain is common, some individuals may experience weight loss as the body fails to utilize glucose properly," says Dr. Satish Koul, Senior Director & Unit Head ...