Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items

A thermometric titration
In association with Nuffield Foundation
- No comments
Use this class practical to practise locating the end-point of a titration by measuring the temperature change
In this experiment, students titrate sodium hydroxide solution with hydrochloric acid. By measuring the temperature change each time a portion of acid is added, students can determine the end-point of the titration, indicated by the highest temperature. They then use this information to calculate the concentration of the hydrochloric acid.
The practical takes about one hour, and is best carried out individually or in pairs.
- Eye protection (goggles)
- Thermometer, 0–100 °C (see note 5 below)
- Two insulated (polystyrene) cups
- Beaker, 250 cm 3
- Burette, 50 cm 3
- Burette stand
- Clamp and stand (optional)
- Cork, one-holed, to fit thermometer (optional)
- Pipette, 20 cm 3 or 25 cm 3
- Pipette safety filler
- Hydrochloric acid, 2.00 M (IRRITANT), about 75 cm 3
- Sodium hydroxide solution, 1.50 M (CORROSIVE), about 30 cm 3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Hydrochloric acid, HCl(aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043. This concentration is necessary to achieve a reasonable change in temperature. The concentration of the hydrochloric acid should not be indicated on bottle available to the students.
- Sodium hydroxide solution, NaOH(aq), (CORROSIVE at concentration used) – see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085. This concentration is necessary to achieve a reasonable change in temperature. The concentration of the sodium hydroxide should be indicated on bottle available to the students.
- Instead of using the thermometer to stir the titration mixture, it could be clamped in position in a cork, as shown in the diagram, and the mixture swirled after each addition of acid. Alternatively, a temperature sensor attached to a computer can be used in place of a thermometer. Data logging software could then be used to provide a detailed plot of the readings.
- Stand an insulated cup in a beaker for support.
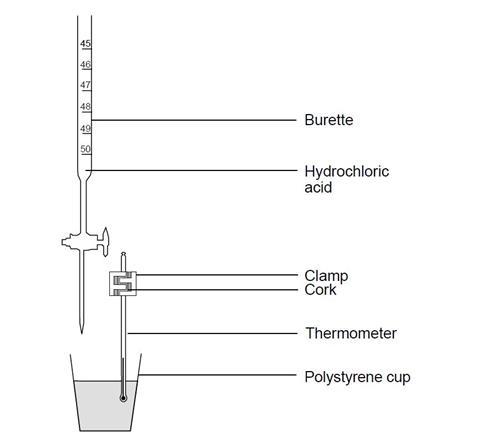
Source: Royal Society of Chemistry
In this thermometric titration, students can determine the end-point of the titration using the highest temperature recorded during the experiment
- Using a pipette and safety filler, transfer 20 cm 3 (or 25 cm 3 ) of the sodium hydroxide solution into the cup, and measure the steady temperature.
- Using the burette, add a small portion (3–5 cm 3 ) of dilute hydrochloric acid to the solution in the cup, noting down the actual volume reading. Stir by swirling the cup and measure the highest temperature reached.
- Immediately add a second small portion of the dilute hydrochloric acid, stir, and again measure the highest temperature and note down the volume reading.
- Continue in this way until there are enough readings to decide the maximum temperature reached during this experiment. You will need to add at least 30 cm 3 of the acid.
- Plot a graph of temperature against the volume of acid added, and use extrapolation of the two sections of the graph to deduce the maximum temperature reached without heat loss.
- Use your results to calculate the concentration of the hydrochloric acid.
Teaching notes
The main concern in this experiment is the heat loss. If possible, a lid should be used. More reliable results can be achieved using two polystyrene cups (one inside the other).
With abler or older students, it is possible to discuss the extrapolation of the cooling curve to estimate the maximum temperature reached without heat loss. Creative Chemistry provide a resource on thermometric titration which includes an example of a typical plot of temperature vs volume of acid for this experiment, as well as the use of extrapolation to determine the maximum temperature change.
To reinforce the theory involved here, an indicator could also be used to show that the end-point really did occur at the highest temperature.
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology .
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists .
© Nuffield Foundation and the Royal Society of Chemistry
- 14-16 years
- 16-18 years
- Practical experiments
- Acids and bases
- Quantitative chemistry and stoichiometry
- Reactions and synthesis
Specification
- (e) simple procedures to determine enthalpy changes
- determine the enthalpy changes for combustion and neutralisation using simple apparatus; and
- 2.8.6 recall experimental methods to determine enthalpy changes;
- 1.8.11 investigate the temperature change during neutralisation and demonstrate understanding that neutralisation reactions are exothermic (heat is given out);
- 1.8.10 investigate the temperature change during neutralisation and demonstrate understanding that neutralisation reactions are exothermic (heat is given out);
Related articles


Demonstrations with dry ice
2024-08-27T06:00:00Z By Declan Fleming
Explore changes of state and neutralisation reactions with this trio of demonstrations using solid carbon dioxide

Help learners master equilibrium and reversible reactions
2024-06-24T06:59:00Z By Emma Owens
Use this poster, fact sheet and storyboard activity to ensure your 14–16 students understand dynamic equilibrium

Non-burning paper: investigate the fire triangle and conditions for combustion
2024-06-10T05:00:00Z By Declan Fleming
Use this reworking of the classic non-burning £5 note demonstration to explore combustion with learners aged 11–16 years
No comments yet
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud

IMAGES
VIDEO