Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 25 May 2023

Prospects and challenges of green ammonia synthesis
- Dongpei Ye ORCID: orcid.org/0000-0003-0091-6994 1 &
- Shik Chi Edman Tsang ORCID: orcid.org/0000-0002-8796-3146 1
Nature Synthesis volume 2 , pages 612–623 ( 2023 ) Cite this article
5218 Accesses
87 Citations
25 Altmetric
Metrics details
- Chemical hydrogen storage
- Heterogeneous catalysis
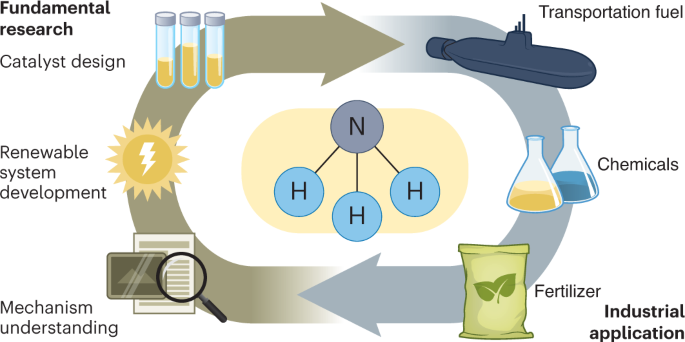
Ammonia is a chemical commodity in high demand, owing to its use in agriculture as well as its potential as a chemical vector for renewable energy storage and transportation. At present, ammonia synthesis consumes 1–2% of the world’s total energy output while producing 1% of the world’s total carbon emissions. Thus, the development of greener synthetic routes to ammonia is urgently required. In this Review, we discuss the progress and challenges in regard to the technological and economic aspects of various routes to green ammonia synthesis. Fundamental mechanisms, including the classical N 2 dissociative process, the newly identified associative process for catalytic N 2 conversion to NH 3 under milder conditions and the chemical looping pathway, are discussed to guide novel catalyst designs. In particular, associative N 2 activation can be achieved at low pressure, which is more adaptable for coupling to renewable energy (such as solar, wind or tidal), offering a new industrial production route to green ammonia. Additional possibilities for direct large-scale green ammonia synthesis through electrochemical and photochemical approaches are also discussed. Finally, a scaleup roadmap for ammonia synthesis is described alongside recent industrial developments, highlighting the rapid evolution and prosperous future of green ammonia generation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency
The why and how of NO x electroreduction to ammonia
Steering from electrochemical denitrification to ammonia synthesis
Bell, T. E. & Torrente-Murciano, L. H 2 production via ammonia decomposition using non-noble metal catalysts: a review. Top. Catal. 59 , 1438–1457 (2016).
CAS Google Scholar
Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrog. Energy 44 , 18179–18192 (2019).
Smith, C., Hill, A. K. & Torrente-Murciano, L.Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 13 , 331–344 (2020).
Google Scholar
Ravi, M. & Makepeace, J. W.Facilitating green ammonia manufacture under milder conditions: what do heterogeneous catalyst formulations have to offer. Chem. Sci. 13 , 890–908 (2022).
CAS PubMed Google Scholar
Bañares-Alcántara, R. et al. Analysis of Islanded Ammonia-based Energy Storage Systems (Univ. Oxford, 2015).
Wang, Q., Guo, J. & Chen, P. Recent progress towards mild-condition ammonia synthesis. J. Energy Chem. 36 , 25–36 (2019).
Ghavam, S., Vahdati, M., Wilson, I. A. G. & Styring, P. Sustainable ammonia production processes. Front. Energy Res. 9 , 580808 (2021).
Bellenger, R., Darnajoux, X., Zhang, A. M. L. & Kraepiel, J. P. Biological nitrogen fixation by alternative nitrogenases in terrestrial ecosystems: a review. Biogeochemistry 149 , 53–73 (2020).
Hywind Scotland (Equinor, 2022); https://www.equinor.com/energy/hywind-scotland
Offshore Solutions (Siemens Energy Global, 2022); https://www.siemens-energy.com/global/en/offerings/industrial-applications/oil-gas/offshore-solutions.html
Foster, S. L. et al. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 1 , 490–500 (2018).
Walter, M. D. Ammonia formation revisited. Nat. Chem. 14 , 12–13 (2021).
Mortensen, J. J., Hansen, L. B., Hammer, B. & Nørskov, J. K. et al. Nitrogen adsorption and dissociation on Fe(111). J. Catal. 182 , 479–488 (1999).
Ertl, G. Reactions at surfaces: from atoms to complexity. Angew. Chem. Int. Ed. 47 , 3524–3535 (2007).
Qian, J., An, Q., Fortunelli, A., Nielsen, R. J. & Goddard, W. A. Reaction mechanism and kinetics for ammonia synthesis on the Fe(111) surface. J. Am. Chem. Soc. 140 , 6288–6297 (2018).
Lin, R. J., Li, F. Y. & Chen, H. L. Computational investigation on adsorption and dissociation of the NH 3 molecule on the Fe(111) surface. J. Phys. Chem. C 115 , 521–528 (2011).
Egeberg, R. C. et al. N 2 dissociation on Fe(110) and Fe/Ru(0001): what is the role of steps? Surf. Sci. 491 , 183–194 (2001).
Medford, A. J. et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 328 , 36–42 (2015).
Rod, T. H., Logadottir, A. & Nørskov, J. K. Ammonia synthesis at low temperatures. J. Chem. Phys. 112 , 5343–5347 (2000).
Humphreys, J., Lan, R. & Tao, S. Development and recent progress on ammonia synthesis catalysts for Haber–Bosch process. Adv. Energy Sustain. Res. 2 , 2000043 (2021).
Jacobsen, C. J. H. et al. Catalyst design by interpolation in the periodic table: bimetallic ammonia synthesis catalysts. J. Am. Chem. Soc. 123 , 8404–8405 (2001).
Fang, H. et al. Challenges and opportunities of Ru-based catalysts toward the synthesis and utilization of ammonia. ACS Catal. 12 , 3938–3954 (2022).
Arnaiz del Pozo, C. & Cloete, S. Techno-economic assessment of blue and green ammonia as energy carriers in a low-carbon future. Energy Convers. Manag. 255 , 115312 (2022).
Sato, K. & Nagaoka, K. Boosting ammonia synthesis under mild reaction conditions by precise control of the basic oxide–Ru interface. Chem. Lett. 50 , 687–696 (2021).
Sato, K. et al. Surface dynamics for creating highly active Ru sites for ammonia synthesis: accumulation of a low-crystalline, oxygen-deficient nanofraction. ACS Sustain. Chem. Eng. 8 , 2726–2734 (2020).
Lin, B. et al. Morphology effect of ceria on the catalytic performances of Ru/CeO 2 catalysts for ammonia synthesis. Ind. Eng. Chem. Res. 57 , 9127–9135 (2018).
Marakatti, V. S. & Gaigneaux, E. M. Recent advances in heterogeneous catalysis for ammonia synthesis. ChemCatChem 12 , 5838–5857 (2020).
Feng, J. et al. Sub-nanometer Ru clusters on ceria nanorods as efficient catalysts for ammonia synthesis under mild conditions. ACS Sustain. Chem. Eng. 10 , 10181–10191 (2022).
Wu, S. et al. Removal of hydrogen poisoning by electrostatically polar MgO support for low-pressure NH 3 synthesis at a high rate over the Ru catalyst. ACS Catal. 10 , 5614–5622 (2020).
Wu, S. et al. Rapid interchangeable hydrogen, hydride, and proton species at the interface of transition metal atom on oxide surface. J. Am. Chem. Soc. 143 , 9105–9112 (2021).
Kitano, M. et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 4 , 934–940 (2012).
Kitano, M. et al. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat. Commun. 6 , 6731 (2015).
Kammert, J. et al. Nature of reactive hydrogen for ammonia synthesis over a Ru/C12A7 electride catalyst. J. Am. Chem. Soc. 142 , 7655–7667 (2020).
Wu, J. et al. Intermetallic electride catalyst as a platform for ammonia synthesis. Angew. Chem. Int. Ed. 58 , 825–829 (2019).
Gong, Y. et al.LaRuSi electride disrupts the scaling relations for ammonia synthesis. Chem. Mater. 34 , 1677–1685 (2022).
Zhang, X. et al. Synergizing surface hydride species and Ru clusters on Sm 2 O 3 for efficient ammonia synthesis. ACS Catal. 12 , 2178–2190 (2022).
García-García, F. R., Guerrero-Ruiz, A. & Rodríguez-Ramos, I. Role of B5-type sites in Ru catalysts used for the NH 3 decomposition reaction. Top. Catal. 52 , 758–764 (2009).
Shetty, S., Jansen, A. P. J. & Van Santen, R. A. Active sites for N 2 dissociation on ruthenium. J. Phys. Chem. C 112 , 17768–17771 (2008).
Wang, L., Chen, J., Ge, L., Rudolph, V. & Zhu, Z. Difference in the cooperative interaction between carbon nanotubes and Ru particles loaded on their internal/external surface. RSC Adv. 3 , 12641–12647 (2013).
Li, L. et al. Size sensitivity of supported Ru catalysts for ammonia synthesis: from nanoparticles to subnanometric clusters and atomic clusters. Chem 8 , 749–768 (2022).
Zhou, Y. et al. Unraveling the size-dependent effect of Ru-based catalysts on ammonia synthesis at mild conditions. J. Catal. 404 , 501–511 (2021).
Zeinalipour-Yazdi, C. D., Richard, C., Catlow, A., Hargreaves, J. S. J. & Laassiri, S. A comparative analysis of the mechanisms of ammonia synthesis on various catalysts using density functional theory. R. Soc. Open Sci. 8 , 210952 (2021).
CAS PubMed PubMed Central Google Scholar
Kojima, R. & Aika, K. I. Cobalt molybdenum bimetallic nitride catalysts for ammonia synthesis. Chem Lett. 29 , 514–515 (2003).
Zeinalipour-Yazdi, C. D., Hargreaves, J. S. J., Richard, C. & Catlow, A. Low-T mechanisms of ammonia synthesis on Co 3 Mo 3 N. J. Phys. Chem. C 122 , 6078–6082 (2018).
Zeinalipour-Yazdi, C. D., Hargreaves, J. S. J., Laassiri, S., Richard, C. & Catlow, A. The integration of experiment and computational modelling in heterogeneously catalysed ammonia synthesis over metal nitrides. Phys. Chem. Chem. Phys. 20 , 21803–21808 (2018).
Ye, T.-N. et al. Vacancy-enabled N 2 activation for ammonia synthesis on an Ni-loaded catalyst. Nature 583 , 391–395 (2020).
Ye, T.-N. et al. Contribution of nitrogen vacancies to ammonia synthesis over metal nitride catalysts. J. Am. Chem. Soc. 142 , 14374–14383 (2020).
Wang, Q. et al. Ternary ruthenium complex hydrides for ammonia synthesis via the associative mechanism. Nat. Catal. 4 , 959–967 (2021).
Hattori, M., Iijima, S., Nakao, T., Hosono, H. & Hara, M. Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C. Nat. Commun. 11 , 2001 (2020).
Liu, J.-C. et al. Heterogeneous Fe 3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 9 , 1610 (2018).
PubMed PubMed Central Google Scholar
Zheng, J. et al. Efficient non-dissociative activation of dinitrogen to ammonia over lithium-promoted ruthenium nanoparticles at low pressure. Angew. Chem. Int. Ed. 58 , 17335–17341 (2019).
Wang, X. et al. Atomically dispersed Ru catalyst for low-temperature nitrogen activation to ammonia via an associative mechanism. ACS Catal. 10 , 9504–9514 (2020).
Ye, T.-N. et al. Dissociative and associative concerted mechanism for ammonia synthesis over Co-based catalyst. J. Am. Chem. Soc. 143 , 12857–12866 (2021).
Zhou, Y. et al. Integrating dissociative and associative routes for efficient ammonia synthesis over a TiCN-promoted Ru-based catalyst. ACS Catal. 12 , 2651–2660 (2022).
Lai, Q. et al. Chemical looping based ammonia production—a promising pathway for production of the noncarbon fuel. Sci. Bull. 67 , 2124–2138 (2022).
Gao, W. et al. Production of ammonia via a chemical looping process based on metal imides as nitrogen carriers. Nat. Energy 3 , 1067–1075 (2018).
Yan, H. et al. Lithium palladium hydride promotes chemical looping ammonia synthesis mediated by lithium imide and hydride. J. Phys. Chem. C 125 , 6716–6722 (2021).
Yang, S. et al. Molybdenum-based nitrogen carrier for ammonia production via a chemical looping route. Appl. Catal. B Environ. 312 , 121404 (2022).
Tagawa, K., Gi, H., Shinzato, K., Miyaoka, H. & Ichikawa, T. Improvement of kinetics of ammonia synthesis at ambient pressure by the chemical looping process of lithium hydride. J. Phys. Chem. C 126 , 2403–2409 (2022).
Xiong, C. et al. High thermal stability Si–Al based N-carrier for efficient and stable chemical looping ammonia generation. Appl. Energy 323 , 119519 (2022).
Pereira, R. J. L., Hu, W. & Metcalfe, I. S. Impact of gas–solid reaction thermodynamics on the performance of a chemical looping ammonia synthesis process. Energy Fuels 36 , 9757–9767 (2022).
Jain, M., Muthalathu, R. & Wu, X. Y. Electrified ammonia production as a commodity and energy storage medium to connect the food, energy, and trade sectors. iScience 25 , 104724 (2022).
Lazouski, N., Chung, M., Williams, K., Gala, M. L. & Manthiram, K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal. 3 , 463–469 (2020).
Suryanto, B. H. R. et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2 , 290–296 (2019).
Wang, M. et al. Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential. Nat. Commun. 10 , 341 (2019).
Chen, G. F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5 , 605–613 (2020).
Wu, Z. Y. et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 12 , 2870 (2021).
Shen, H. et al. Electrochemical ammonia synthesis: mechanistic understanding and catalyst design. Chem 7 , 1708–1754 (2021).
Lazouski, N., Schiffer, Z. J., Williams, K. & Manthiram, K. Understanding continuous lithium-mediated electrochemical nitrogen reduction. Joule 3 , 1127–1139 (2019).
Suryanto, B. H. R. et al. Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle. Science 372 , 1187–1191 (2021).
Du, H. L. et al. Electroreduction of nitrogen with almost 100% current-to-ammonia efficiency. Nature 609 , 722–727 (2022).
Li, K. et al. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen. Science 374 , 1593–1597 (2021).
Li, S. et al. Electrosynthesis of ammonia with high selectivity and high rates via engineering of the solid–electrolyte interphase. Joule 6 , 2083–2101 (2022).
Murakami, T., Nohira, T., Goto, T., Ogata, Y. H. & Ito, Y. Electrolytic ammonia synthesis from water and nitrogen gas in molten salt under atmospheric pressure. Electrochim. Acta 50 , 5423–5426 (2005).
McPherson, I. J. et al. The feasibility of electrochemical ammonia synthesis in molten LiCl–KCl eutectics. Angew. Chem. Int. Ed. 58 , 17433–17441 (2019).
McEnaney, J. M. et al. Ammonia synthesis from N 2 and H 2 O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 10 , 1621–1630 (2017).
Wu, S., Salmon, N., Li, M. M. J., Bañares-Alcántara, R. & Tsang, S. C. E. Energy decarbonization via green H 2 or NH 3 ? ACS Energy Lett. 7 , 1021–1033 (2022).
Biswas, S. S., Saha, A. & Eswaramoorthy, M. Facts or artifacts: pitfalls in quantifying sub-ppm levels of ammonia produced from electrochemical nitrogen reduction. ACS Omega 7 , 1874–1882 (2022).
Andersen, S. Z. et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 570 , 504–508 (2019).
Han, Q., Jiao, H., Xiong, L. & Tang, J. Progress and challenges in photocatalytic ammonia synthesis. Mater. Adv. 2 , 564–581 (2021).
Zhang, G. S vacancies act as a bridge to promote electron injection from Z-scheme heterojunction to nitrogen molecule for photocatalytic ammonia synthesis. Chem. Eng. J. 433 , 133670 (2022).
Han, Q. et al. Rational design of high-concentration Ti 3 + in porous carbon-doped TiO 2 nanosheets for efficient photocatalytic ammonia synthesis. Adv. Mater. 33 , 2008180 (2021).
Yin, H. et al. Dual active centers bridged by oxygen vacancies of ruthenium single-atom hybrids supported on molybdenum oxide for photocatalytic ammonia synthesis. Angew. Chem. Int. Ed. 61 , e202114242 (2022).
Liu, G. et al. Boosting photocatalytic nitrogen reduction to ammonia by dual defective -C≡N and K-doping sites on graphitic carbon nitride nanorod arrays. Appl. Catal. B Environ. 317 , 121752 (2022).
Kim, S., Park, Y., Kim, J., Pabst, T. P. & Chirik, P. J. Ammonia synthesis by photocatalytic hydrogenation of a N 2 -derived molybdenum nitride. Nat. Synth. 1 , 297–303 (2022).
Wang, M. et al. Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber–Bosch processes? Energy Environ. Sci. 14 , 2535–2548 (2021).
Wang, T. & Abild-Pedersen, F. Achieving industrial ammonia synthesis rates at near-ambient conditions through modified scaling relations on a confined dual site. Proc. Natl Acad. Sci. USA 118 , 2106527118 (2021).
Soloveichik, G. Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat. Catal. 2 , 377–380 (2019).
Grigoriev, S. A., Fateev, V. N., Bessarabov, D. G. & Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrog. Energy 45 , 26036–26058 (2020).
Morlanés, N. et al. A technological roadmap to the ammonia energy economy: current state and missing technologies. Chem. Eng. J. 408 , 127310 (2021).
MacFarlane, D. R. et al. A roadmap to the ammonia economy. Joule 4 , 1186–1205 (2020).
Ye, L., Nayak-Luke, R., Bañares-Alcántara, R. & Tsang, E. Reaction: ‘green’ ammonia production. Chem 3 , 712–714 (2017).
Hansen, J. B., Han, P. Green Ammonia by Haldor Topsoe (Department of Energy, 2021); https://www.energy.gov/sites/default/files/2021-08/4-green-ammonia-haldor-topsoe.pdf
Hansen, J. B. High Efficient Ammonia Synthesis Systems (Ammonia Energy Association, 2019); https://www.ammoniaenergy.org/wp-content/uploads/2021/06/1.1-John-Hansen-NH3-Event-Melbourne-Topsoe-2019.pdf
Small-Scale Green Ammonia Plants Open up New Storage Possibilities for Wind and Solar Power (ThyssenKrupp Industrial Solutions, 2022); https://insights.thyssenkrupp-industrial-solutions.com/story/small-scale-green-ammonia-plants-open-up-new-storage-possibilities-for-wind-and-solar-power/
Yara Selects Linde Engineering to Build Electrolysis Plant at Porsgrunn (Ammonia Energy Association, 2022); https://www.ammoniaenergy.org/articles/yara-selects-linde-engineering-to-build-electrolysis-plant-at-porsgrunn/
Renewable Ammonia in Vietnam (Ammonia Energy Association, 2022); https://www.ammoniaenergy.org/articles/renewable-ammonia-in-vietnam/
ABS Publishes Offshore Production of Green Hydrogen (American Bureau of Shipping, 2022); https://absinfo.eagle.org/acton/media/16130/offshore-production-of-green-hydrogen
The P2XFloater TM (H2CARRIER, 2022); https://www.h2carrier.com/the-p2x-floater
Gerretsen, I. The floating solar panels that track the Sun. BBC Future (18 November 2022); https://www.bbc.com/future/article/20221116-the-floating-solar-panels-that-track-the-sun
Hong, J., Prawer, S. & Murphy, A. B. Plasma catalysis as an alternative route for ammonia production: status, mechanisms, and prospects for progress. ACS Sustain. Chem. Eng. 6 , 15–31 (2017).
Engelmann, Y. et al. Plasma catalysis for ammonia synthesis: a microkinetic modeling study on the contributions of Eley–Rideal reactions. ACS Sustain. Chem. Eng. 9 , 13151–13163 (2021).
Lee, K. et al. Techno-economic performances and life cycle greenhouse gas emissions of various ammonia production pathways including conventional, carbon-capturing, nuclear-powered, and renewable production. Green Chem. 24 , 4830–4844 (2022).
Mills, A. et al. Qatar to build world’s largest ‘blue’ ammonia plant—QatarEnergy. Reuters (1 September 2022); https://www.reuters.com/business/energy/qatar-build-worlds-largest-blue-ammonia-plant-qatarenergy-ceo-2022-08-31/
Frohlke, U. Topsoe and First Ammonia launch zero emission ammonia production with the world’s largest reservation of electrolyzer capacity. Topsoe (14 September 2022); https://blog.topsoe.com/topsoe-and-first-ammonia
Download references
Acknowledgements
We acknowledge financial support from the Engineering and Physical Sciences Research Council Divisional Cooperative Awards in Science and Technology Conversion Incentivisation Scheme and OXGRIN. Credit: fertilizer/mechanical understanding icons in the graphical abstract, Flaticon.com .
Author information
Authors and affiliations.
Wolfson Catalysis Centre, Department of Chemistry, University of Oxford, Oxford, UK
Dongpei Ye & Shik Chi Edman Tsang
You can also search for this author in PubMed Google Scholar
Contributions
S.C.E.T. and D.Y. contributed to the discussion and wrote the manuscript.
Corresponding author
Correspondence to Shik Chi Edman Tsang .
Ethics declarations
Competing interests.
The authors declare no competing interests.

Peer review
Peer review information.
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Ye, D., Tsang, S.C.E. Prospects and challenges of green ammonia synthesis. Nat. Synth 2 , 612–623 (2023). https://doi.org/10.1038/s44160-023-00321-7
Download citation
Received : 22 November 2022
Accepted : 14 April 2023
Published : 25 May 2023
Issue Date : July 2023
DOI : https://doi.org/10.1038/s44160-023-00321-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Isolating cu-zn active-sites in ordered intermetallics to enhance nitrite-to-ammonia electroreduction.
- Yongwen Tan
Nature Communications (2024)
In-situ electrochemical reconstruction and modulation of adsorbed hydrogen coverage in cobalt/ruthenium-based catalyst boost electroreduction of nitrate to ammonia
- Thomas Quast
- Wolfgang Schuhmann
From sustainable feedstocks to microbial foods
- Kyeong Rok Choi
- Seok Yeong Jung
- Sang Yup Lee
Nature Microbiology (2024)
Crop migration and environmental consequences
- Shu Kee Lam
Nature Food (2024)
Metal nitrides as electrocatalysts in green ammonia synthesis
- A. Januszewska-Kubsik
- S. Podsiadło
- M. Siekierski
Applied Physics A (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
The Haber process is the main industrial procedure for producing ammonia by reacting atmospheric nitrogen with hydrogen using a catalyst. It was developed by Fritz Haber and Carl Bosch in the early 20th century and is essential for fertilizers and munitions.
Learn how ammonia is produced worldwide from nitrogen and hydrogen using various processes, such as the Haber-Bosch process, the Frank-Caro process, and the Birkeland-Eyde process. Find out the environmental and sustainability issues related to ammonia production and its uses.
This review article discusses the recent advances in ammonia synthesis processes, catalytic materials, and applications as a clean energy vector. It compares the conventional Haber-Bosch technology with renewable energy-based methods and proposes a future end-use vision for ammonia.
Taking electrocatalytic ammonia synthesis as an example, slightly less energy is needed to produce ammonia than for the Haber-Bosch synthesis 8. However, most catalysts show low efficiencies and ...
N 2 -H 2 activation to ammonia—also referred to as indirect ammonia synthesis or the Haber-Bosch reaction—is the reduction of dinitrogen to ammonia with the aid of hydrogen. Due to the ...
Learn about the process of producing ammonia, a chemical compound used in various industries, and the emerging technologies to reduce its carbon footprint. Explore chapters and articles on ammonia synthesis, catalysis, electrolysis, and more.
This article delves into the latest advancements in ammonia synthesis, transitioning from the energy-intensive Haber-Bosch process to more sustainable strategies. It emphasizes the gas-solid reactions based on N 2 and H 2. The aim of this paper is to illustrate how to overcome the thermodynamic and kinetic challenges and to outline the future ...
Ammonia synthesis is an exothermic reaction (negative enthalpy change), and it occurs spontaneously at low temperatures (negative entropy change). Although it is favored at room temperature, the reaction rate at which the reaction occurs at room temperature is too slow to be applicable for at an industrial scale. In order to increase the ...
Haber-Bosch process, method of directly synthesizing ammonia from hydrogen and nitrogen, developed by the German physical chemist Fritz Haber. It was the first industrial chemical process to use high pressure for a chemical reaction. Learn more about the Haber-Bosch process in this article.
Ammonia (NH 3) is the second most produced chemical globally, with an annual production of 183 million metric tons ().It is primarily used for energy transport, fertilizer production, and the synthesis of fine chemicals (2, 3).The Haber-Bosch process is the predominant method for ammonia synthesis, using methane (CH 4) as a hydrogen source and an iron-based catalyst to break the N≡N triple ...