Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 11 November 2022

Error, reproducibility and uncertainty in experiments for electrochemical energy technologies
- Graham Smith ORCID: orcid.org/0000-0003-0713-2893 1 &
- Edmund J. F. Dickinson ORCID: orcid.org/0000-0003-2137-3327 1
Nature Communications volume 13 , Article number: 6832 ( 2022 ) Cite this article
10k Accesses
9 Citations
22 Altmetric
Metrics details
- Electrocatalysis
- Electrochemistry
- Materials for energy and catalysis
The authors provide a metrology-led perspective on best practice for the electrochemical characterisation of materials for electrochemical energy technologies. Such electrochemical experiments are highly sensitive, and their results are, in practice, often of uncertain quality and challenging to reproduce quantitatively.
A critical aspect of research on electrochemical energy devices, such as batteries, fuel cells and electrolysers, is the evaluation of new materials, components, or processes in electrochemical cells, either ex situ, in situ or in operation. For such experiments, rigorous experimental control and standardised methods are required to achieve reproducibility, even on standard or idealised systems such as single crystal platinum 1 . Data reported for novel materials often exhibit high (or unstated) uncertainty and often prove challenging to reproduce quantitatively. This situation is exacerbated by a lack of formally standardised methods, and practitioners with less formal training in electrochemistry being unaware of best practices. This limits trust in published metrics, with discussions on novel electrochemical systems frequently focusing on a single series of experiments performed by one researcher in one laboratory, comparing the relative performance of the novel material against a claimed state-of-the-art.
Much has been written about the broader reproducibility/replication crisis 2 and those reading the electrochemical literature will be familiar with weakly underpinned claims of “outstanding” performance, while being aware that comparisons may be invalidated by measurement errors introduced by experimental procedures which violate best practice; such issues frequently mar otherwise exciting science in this area. The degree of concern over the quality of reported results is evidenced by the recent decision of several journals to publish explicit experimental best practices 3 , 4 , 5 , reporting guidelines or checklists 6 , 7 , 8 , 9 , 10 and commentary 11 , 12 , 13 aiming to improve the situation, including for parallel theoretical work 14 .
We write as two electrochemists who, working in a national metrology institute, have enjoyed recent exposure to metrology: the science of measurement. Metrology provides the vocabulary 15 and mathematical tools 16 to express confidence in measurements and the comparisons made between them. Metrological systems and frameworks for quantification underpin consistency and assurance in all measurement fields and formal metrology is an everyday consideration for practical and academic work in fields where accurate measurements are crucial; we have found it a useful framework within which to evaluate our own electrochemical work. Here, rather than pen another best practice guide, we aim, with focus on three-electrode electrochemical measurements for energy material characterisation, to summarise some advice that we hope helps those performing electrochemical experiments to:
avoid mistakes and minimise error
report in a manner that facilitates reproducibility
consider and quantify uncertainty
Minimising mistakes and error
Metrology dispenses with nebulous concepts such as performance and instead requires scientists to define a specific measurand (“the quantity intended to be measured”) along with a measurement model ( ”the mathematical relation among all quantities known to be involved in a measurement”), which converts the experimental indicators into the measurand 15 . Error is the difference between the reported value of this measurand and its unknowable true value. (Note this is not the formal definition, and the formal concepts of error and true value are not fully compatible with measurement concepts discussed in this article, but we retain it here—as is common in metrology tuition delivered by national metrology institutes—for pedagogical purposes 15 ).
Mistakes (or gross errors) are those things which prevent measurements from working as intended. In electrochemistry the primary experimental indicator is often current or voltage, while the measurand might be something simple, like device voltage for a given current density, or more complex, like a catalyst’s turnover frequency. Both of these are examples of ‘method-defined measurands’, where the results need to be defined in reference to the method of measurement 17 , 18 (for example, to take account of operating conditions). Robust experimental design and execution are vital to understand, quantify and minimise sources of error, and to prevent mistakes.
Contemporary electrochemical instrumentation can routinely offer a current resolution and accuracy on the order of femtoamps; however, one electron looks much like another to a potentiostat. Consequently, the practical limit on measurements of current is the scientist’s ability to unambiguously determine what causes the observed current. Crucially, they must exclude interfering processes such as modified/poisoned catalyst sites or competing reactions due to impurities.
As electrolytes are conventionally in enormous excess compared to the active heterogeneous interface, electrolyte purity requirements are very high. Note, for example, that a perfectly smooth 1 cm 2 polycrystalline platinum electrode has on the order of 2 nmol of atoms exposed to the electrolyte, so that irreversibly adsorbing impurities present at the part per billion level (nmol mol −1 ) in the electrolyte may substantially alter the surface of the electrode. Sources of impurities at such low concentration are innumerable and must be carefully considered for each experiment; impurity origins for kinetic studies in aqueous solution have been considered broadly in the historical literature, alongside a review of standard mitigation methods 19 . Most commercial electrolytes contain impurities and the specific ‘grade’ chosen may have a large effect; for example, one study showed a three-fold decrease in the specific activity of oxygen reduction catalysts when preparing electrolytes with American Chemical Society (ACS) grade acid rather than a higher purity grade 20 . Likewise, even 99.999% pure hydrogen gas, frequently used for sparging, may contain more than the 0.2 μmol mol −1 of carbon monoxide permitted for fuel cell use 21 .
The most insidious impurities are those generated in situ. The use of reference electrodes with chloride-containing filling solutions should be avoided where chloride may poison catalysts 22 or accelerate dissolution. Similarly, reactions at the counter electrode, including dissolution of the electrode itself, may result in impurities. This is sometimes overlooked when platinum counter electrodes are used to assess ‘platinum-free’ electrocatalysts, accidentally resulting in performance-enhancing contamination 23 , 24 ; a critical discussion on this topic has recently been published 25 . Other trace impurity sources include plasticisers present in cells and gaskets, or silicates from the inappropriate use of glass when working with alkaline electrolytes 26 . To mitigate sensitivity to impurities from the environment, cleaning protocols for cells and components must be robust 27 . The use of piranha solution or similarly oxidising solution followed by boiling in Type 1 water is typical when performing aqueous electrochemistry 20 . Cleaned glassware and electrodes are also routinely stored underwater to prevent recontamination from airborne impurities.
The behaviour of electronic hardware used for electrochemical experiments should be understood and considered carefully in interpreting data 28 , recognising that the built-in complexity of commercially available digital potentiostats (otherwise advantageous!) is capable of introducing measurement artefacts or ambiguity 29 , 30 . While contemporary electrochemical instrumentation may have a voltage resolution of ~1 μV, its voltage measurement uncertainty is limited by other factors, and is typically on the order of 1 mV. As passing current through an electrode changes its potential, a dedicated reference electrode is often incorporated into both ex situ and, increasingly, in situ experiments to provide a stable well defined reference. Reference electrodes are typically selected from a range of well-known standardised electrode–electrolyte interfaces at which a characteristic and kinetically rapid reversible faradaic process occurs. The choice of reference electrode should be made carefully in consideration of chemical compatibility with the measurement environment 31 , 32 , 33 , 34 . In combination with an electronic blocking resistance, the potential of the electrode should be stable and reproducible. Unfortunately, deviation from the ideal behaviour frequently occurs. While this can often be overlooked when comparing results from identical cells, more attention is required when reporting values for comparison.
In all cases where conversion between different electrolyte–reference electrode systems is required, junction potentials should be considered. These arise whenever there are different chemical conditions in the electrolyte at the working electrode and reference electrode interfaces. Outside highly dilute solutions, or where there are large activity differences for a reactant/product of the electrode reaction (e.g. pH for hydrogen reactions), liquid junction potentials for conventional aqueous ions have been estimated in the range <50 mV 33 . Such a deviation may nonetheless be significant when onset potentials or activities at specific potentials are being reported. The measured potential difference between the working and reference electrode also depends strongly on the geometry of the cell, so cell design is critical. Fig. 1 shows the influence of cell design on potential profiles. Ideally the reference electrode should therefore be placed close to the working electrode (noting that macroscopic electrodes may have inhomogeneous potentials). To minimise shielding of the electric field between counter and working electrode and interruption of mass transport processes, a thin Luggin-Haber capillary is often used and a small separation maintained. Understanding of shielding and edge effects is vital when reference electrodes are introduced in situ. This is especially applicable for analysis of energy devices for which constraints on cell design, due to the need to minimise electrolyte resistance and seal the cell, preclude optimal reference electrode positioning 32 , 35 , 36 .
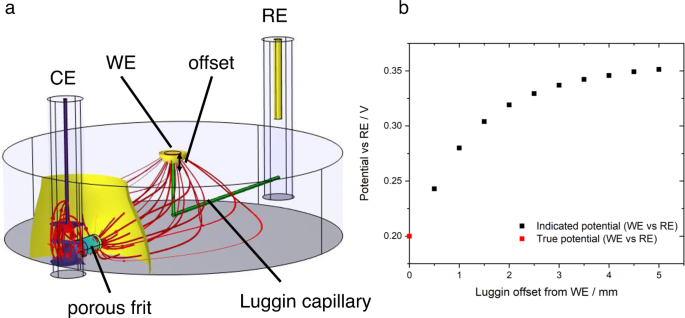
a Illustration (simulated data) of primary (resistive) current and potential distribution in a typical three-electrode cell. The main compartment is cylindrical (4 cm diameter, 1 cm height), filled with electrolyte with conductivity 1.28 S m −1 (0.1 M KCl(aq)). The working electrode (WE) is a 2 mm diameter disc drawing 1 mA (≈ 32 mA cm −2 ) from a faradaic process with infinitely fast kinetics and redox potential 0.2 V vs the reference electrode (RE). The counter electrode (CE) is connected to the main compartment by a porous frit; the RE is connected by a Luggin capillary (green cylinders) whose tip position is offset from the WE by a variable distance. Red lines indicate prevailing current paths; coloured surfaces indicate isopotential contours normal to the current density. b Plot of indicated WE vs RE potential (simulated data). As the Luggin tip is moved away from the WE surface, ohmic losses due to the WE-CE current distribution lead to variation in the indicated WE-RE potential. Appreciable error may arise on an offset length scale comparable to the WE radius.
Quantitative statements about fundamental electrochemical processes based on measured values of current and voltage inevitably rely on models of the system. Such models have assumptions that may be routinely overlooked when following experimental and analysis methods, and that may restrict their application to real-world systems. It is quite possible to make highly precise but meaningless measurements! An often-assumed condition for electrocatalyst analysis is the absence of mass transport limitation. For some reactions, such as the acidic hydrogen oxidation and hydrogen evolution reactions, this state is arguably so challenging to reach at representative conditions that it is impossible to measure true catalyst activity 11 . For example, ex situ thin-film rotating disk electrode measurements routinely fail to predict correct trends in catalyst performance in morphologically complex catalyst layers as relevant operating conditions (e.g. meaningful current densities) are theoretically inaccessible. This topic has been extensively discussed with some authors directly criticising this technique and exploring alternatives 37 , 38 , and others defending the technique’s applicability for ranking catalysts if scrupulous attention is paid to experimental details 39 ; yet, many reports continue to use this measurement technique blindly with no regard for its applicability. We therefore strongly urge those planning measurements to consider whether their chosen technique is capable of providing sufficient evidence to disprove their hypothesis, even if it has been widely used for similar experiments.
The correct choice of technique should be dependent upon the measurand being probed rather than simply following previous reports. The case of iR correction, where a measurement of the uncompensated resistance is used to correct the applied voltage, is a good example. When the measurand is a material property, such as intrinsic catalyst activity, the uncompensated resistance is a source of error introduced by the experimental method and it should carefully be corrected out (Fig. 1 ). In the case that the uncompensated resistance is intrinsic to the measurand—for instance the operating voltage of an electrolyser cell—iR compensation is inappropriate and only serves to obfuscate. Another example is the choice of ex situ (outside the operating environment), in situ (in the operating environment), and operando (during operation) measurements. While in situ or operando testing allows characterisation under conditions that are more representative of real-world use, it may also yield measurements with increased uncertainty due to the decreased possibility for fine experimental control. Depending on the intended use of the measurement, an informed compromise must be sought between how relevant and how uncertain the resulting measurement will be.
Maximising reproducibility
Most electrochemists assess the repeatability of measurements, performing the same measurement themselves several times. Repeats, where all steps (including sample preparation, where relevant) of a measurement are carried out multiple times, are absolutely crucial for highlighting one-off mistakes (Fig. 2 ). Reproducibility, however, is assessed when comparing results reported by different laboratories. Many readers will be familiar with the variability in key properties reported for various systems e.g. variability in the reported electrochemically active surface area (ECSA) of commercial catalysts, which might reasonably be expected to be constant, suggesting that, in practice, the reproducibility of results cannot be taken for granted. As electrochemistry deals mostly with method-defined measurands, the measurement procedure must be standardised for results to be comparable. Variation in results therefore strongly suggests that measurements are not being performed consistently and that the information typically supplied when publishing experimental methods is insufficient to facilitate reproducibility of electrochemical measurements. Quantitative electrochemical measurements require control over a large range of parameters, many of which are easily overlooked or specified imprecisely when reporting data. An understanding of the crucial parameters and methods for their control is often institutional knowledge, held by expert electrochemists, but infrequently formalised and communicated e.g. through publication of widely adopted standards. This creates challenges to both reproducibility and the corresponding assessment of experimental quality by reviewers. The reporting standards established by various publishers (see Introduction) offer a practical response, but it is still unclear whether these will contain sufficiently granular detail to improve the situation.
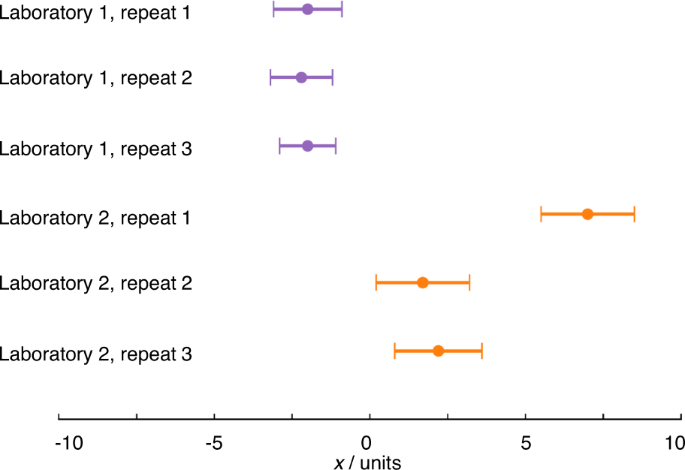
The measurements from laboratory 1 show a high degree of repeatability, while the measurements from laboratory 2 do not. Apparently, a mistake has been made in repeat 1, which will need to be excluded from any analysis and any uncertainty analysis, and/or suggests further repeat measurements should be conducted. The error bars are based on an uncertainty with coverage factor ~95% (see below) so the results from the two laboratories are different, i.e. show poor reproducibility. This may indicate differing experimental practice or that some as yet unidentified parameter is influencing the results.
Besides information typically supplied in the description of experimental methods for publication, which, at a minimum, must detail the materials, equipment and measurement methods used to generate the results, we suggest that a much more comprehensive description is often required, especially where measurements have historically poor reproducibility or the presented results differ from earlier reports. Such an expanded ‘supplementary experimental’ section would additionally include any details that could impact the results: for example, material pre-treatment, detailed electrode preparation steps, cleaning procedures, expected electrolyte and gas impurities, electrode preconditioning processes, cell geometry including electrode positions, detail of junctions between electrodes, and any other fine experimental details which might be institutional knowledge but unknown to the (now wide) readership of the electrochemical literature. In all cases any corrections and calculations used should be specified precisely and clearly justified; these may include determinations of properties of the studied system, such as ECSA, or of the environment, such as air pressure. We highlight that knowledge of the ECSA is crucial for conventional reporting of intrinsic electrocatalyst activity, but is often very challenging to measure in a reproducible manner 40 , 41 .
To aid reproducibility we recommend regularly calibrating experimental equipment and doing so in a way that is traceable to primary standards realising the International System of Units (SI) base units. The SI system ensures that measurement units (such as the volt) are uniform globally and invariant over time. Calibration applies to direct experimental indicators, e.g. loads and potentiostats, but equally to supporting tools such as temperature probes, balances, and flow meters. Calibration of reference electrodes is often overlooked even though variations from ideal behaviour can be significant 42 and, as discussed above, are often the limit of accuracy on potential measurement. Sometimes reports will specify internal calibration against a known reaction (such as the onset of the hydrogen evolution reaction), but rarely detail regular comparisons to a local master electrode artefact such as a reference hydrogen electrode or explain how that artefact is traceable, e.g. through control of the filling solution concentration and measurement conditions. If reference is made to a standardised material (e.g. commercial Pt/C) the specified material should be both widely available and the results obtained should be consistent with prior reports.
Beyond calibration and reporting, the best test of reproducibility is to perform intercomparisons between laboratories, either by comparing results to identical experiments reported in the literature or, more robustly, through participation in planned intercomparisons (for example ‘round-robin’ exercises); we highlight a recent study applied to solid electrolyte characterisation as a topical example 43 . Intercomparisons are excellent at establishing the key features of an experimental method and the comparability of results obtained from different methods; moreover they provide a consensus against which other laboratories may compare themselves. However, performing repeat measurements for assessing repeatability and reproducibility cannot estimate uncertainty comprehensively, as it excludes systematic sources of uncertainty.
Assessing measurement uncertainty
Formal uncertainty evaluation is an alien concept to most electrochemists; even the best papers (as well as our own!) typically report only the standard deviation between a few repeats. Metrological best practice dictates that reported values are stated as the combination of a best estimate of the measurand, and an interval, and a coverage factor ( k ) which gives the probability of the true value being within that interval. For example, “the turnover frequency of the electrocatalyst is 1.0 ± 0.2 s −1 ( k = 2)” 16 means that the scientist (having assumed normally distributed error) is 95% confident that the turnover frequency lies in the range 0.8–1.2 s −1 . Reporting results in such a way makes it immediately clear whether the measurements reliably support the stated conclusions, and enables meaningful comparisons between independent results even if their uncertainties differ (Fig. 3 ). It also encourages honesty and self-reflection about the shortcomings of results, encouraging the development of improved experimental techniques.
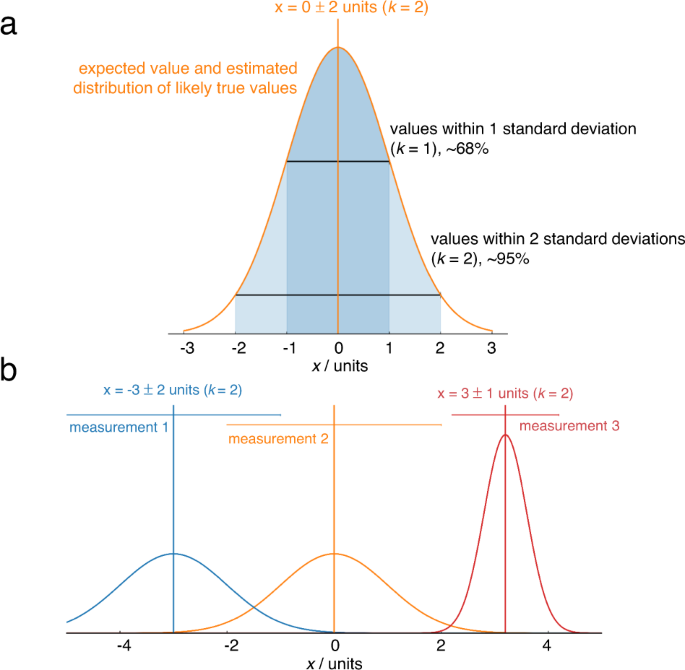
a Complete reporting of a measurement includes the best estimate of the measurand and an uncertainty and the probability the true value falls within the uncertainty reported. Here, the percentages indicate that a normal distribution has been assumed. b Horizontal bars indicate 95% confidence intervals from uncertainty analysis. The confidence intervals of measurements 1 and 2 overlap when using k = 2, so it is not possible to say with 95% confidence that the result of the measurement 2 is higher than measurement 1, but it is possible to say this with 68% confidence, i.e. k = 1. Measurement 3 has a lower uncertainty, so it is possible to say with 95% confidence that the value is higher than measurement 2.
Constructing such a statement and performing the underlying calculations often appears daunting, not least as there are very few examples for electrochemical systems, with pH measurements being one example to have been treated thoroughly 44 . However, a standard process for uncertainty analysis exists, as briefly outlined graphically in Fig. 4 . We refer the interested reader to both accessible introductory texts 45 and detailed step-by-step guides 16 , 46 . The first steps in the process are to state precisely what is being measured—the measurand—and identify likely sources of uncertainty. Even this qualitative effort is often revealing. Precision in the definition of the measurand (and how it is determined from experimental indicators) clarifies the selection of measurement technique and helps to assess its appropriateness; for example, where the measurand relates only to an instantaneous property of a specific physical object, e.g. the current density of a specific fuel cell at 0.65 V following a standardised protocol, we ignore all variability in construction, device history etc. and no error is introduced by the sample. Whereas, when the measurand is a material property, such as turnover frequency of a catalyst material with a defined chemistry and preparation method, variability related to the material itself and sample preparation will often introduce substantial uncertainty in the final result. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or variability in materials and samples. Identifying these sources leads to the design of better-quality experiments. In essence, the subsequent steps in the calculation of uncertainty quantify the uncertainty introduced by each source of error and, by using a measurement model or a sensitivity analysis (i.e. an assessment of how the results are sensitive to variability in input parameters), propagate these to arrive at a final uncertainty on the reported result.
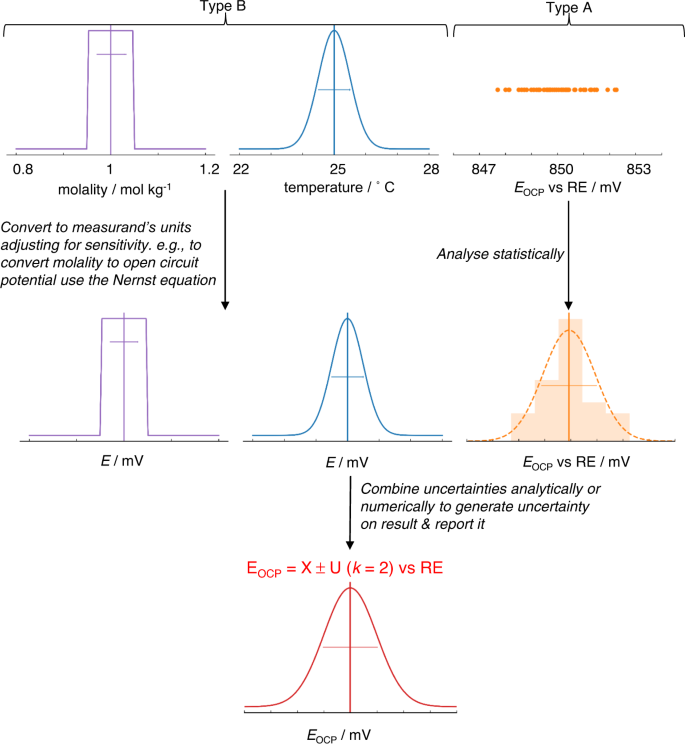
Possible sources of uncertainty are identified, and their standard uncertainty or probability distribution is determined by statistical analysis of repeat measurements (Type A uncertainties) or other evidence (Type B uncertainties). If required, uncertainties are then converted into the same unit as the measurand and adjusted for sensitivity, using a measurement model. Uncertainties are then combined either analytically using a standard approach or numerically to generate an overall estimate of uncertainty for the measurand (as indicated in Fig. 3a ).
Generally, given the historically poor understanding of uncertainty in electrochemistry, we promote increased awareness of uncertainty reporting standards and a focus on reporting measurement uncertainty with a level of detail that is appropriate to the claim made, or the scientific utilisation of the data. For example, where the primary conclusion of a paper relies on demonstrating that a material has the ‘highest ever X’ or ‘X is bigger than Y’ it is reasonable for reviewers to ask authors to quantify how confident they are in their measurement and statement. Additionally, where uncertainties are reported, even with error bars in numerical or graphical data, the method by which the uncertainty was determined should be stated, even if the method is consciously simple (e.g. “error bars indicate the sample standard deviation of n = 3 measurements carried out on independent electrodes”). Unfortunately, we are aware of only sporadic and incomplete efforts to create formal uncertainty budgets for electrochemical measurements of energy technologies or materials, though work is underway in our group to construct these for some exemplar systems.
Electrochemistry has undoubtedly thrived without significant interaction with formal metrology; we do not urge an abrupt revolution whereby rigorous measurements become devalued if they lack additional arcane formality. Rather, we recommend using the well-honed principles of metrology to illuminate best practice and increase transparency about the strengths and shortcomings of reported experiments. From rethinking experimental design, to participating in laboratory intercomparisons and estimating the uncertainty on key results, the application of metrological principles to electrochemistry will result in more robust science.
Climent, V. & Feliu, J. M. Thirty years of platinum single crystal electrochemistry. J. Solid State Electrochem . https://doi.org/10.1007/s10008-011-1372-1 (2011).
Nature Editors and Contributors. Challenges in irreproducible research collection. Nature https://www.nature.com/collections/prbfkwmwvz/ (2018).
Chen, J. G., Jones, C. W., Linic, S. & Stamenkovic, V. R. Best practices in pursuit of topics in heterogeneous electrocatalysis. ACS Catal. 7 , 6392–6393 (2017).
Article CAS Google Scholar
Voiry, D. et al. Best practices for reporting electrocatalytic performance of nanomaterials. ACS Nano 12 , 9635–9638 (2018).
Article CAS PubMed Google Scholar
Wei, C. et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 31 , 1806296 (2019).
Article Google Scholar
Chem Catalysis Editorial Team. Chem Catalysis Checklists Revision 1.1 . https://info.cell.com/introducing-our-checklists-learn-more (2021).
Chatenet, M. et al. Good practice guide for papers on fuel cells and electrolysis cells for the Journal of Power Sources. J. Power Sources 451 , 227635 (2020).
Sun, Y. K. An experimental checklist for reporting battery performances. ACS Energy Lett. 6 , 2187–2189 (2021).
Li, J. et al. Good practice guide for papers on batteries for the Journal of Power Sources. J. Power Sources 452 , 227824 (2020).
Arbizzani, C. et al. Good practice guide for papers on supercapacitors and related hybrid capacitors for the Journal of Power Sources. J. Power Sources 450 , 227636 (2020).
Hansen, J. N. et al. Is there anything better than Pt for HER? ACS Energy Lett. 6 , 1175–1180 (2021).
Article CAS PubMed PubMed Central Google Scholar
Xu, K. Navigating the minefield of battery literature. Commun. Mater. 3 , 1–7 (2022).
Dix, S. T., Lu, S. & Linic, S. Critical practices in rigorously assessing the inherent activity of nanoparticle electrocatalysts. ACS Catal. 10 , 10735–10741 (2020).
Mistry, A. et al. A minimal information set to enable verifiable theoretical battery research. ACS Energy Lett. 6 , 3831–3835 (2021).
Joint Committee for Guides in Metrology: Working Group 2. International Vocabulary of Metrology—Basic and General Concepts and Associated Terms . (2012).
Joint Committee for Guides in Metrology: Working Group 1. Evaluation of measurement data—Guide to the expression of uncertainty in measurement . (2008).
International Organization for Standardization: Committee on Conformity Assessment. ISO 17034:2016 General requirements for the competence of reference material producers . (2016).
Brown, R. J. C. & Andres, H. How should metrology bodies treat method-defined measurands? Accredit. Qual. Assur . https://doi.org/10.1007/s00769-020-01424-w (2020).
Angerstein-Kozlowska, H. Surfaces, Cells, and Solutions for Kinetics Studies . Comprehensive Treatise of Electrochemistry vol. 9: Electrodics: Experimental Techniques (Plenum Press, 1984).
Shinozaki, K., Zack, J. W., Richards, R. M., Pivovar, B. S. & Kocha, S. S. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique. J. Electrochem. Soc. 162 , F1144–F1158 (2015).
International Organization for Standardization: Technical Committee ISO/TC 197. ISO 14687:2019(E)—Hydrogen fuel quality—Product specification . (2019).
Schmidt, T. J., Paulus, U. A., Gasteiger, H. A. & Behm, R. J. The oxygen reduction reaction on a Pt/carbon fuel cell catalyst in the presence of chloride anions. J. Electroanal. Chem. 508 , 41–47 (2001).
Chen, R. et al. Use of platinum as the counter electrode to study the activity of nonprecious metal catalysts for the hydrogen evolution reaction. ACS Energy Lett. 2 , 1070–1075 (2017).
Ji, S. G., Kim, H., Choi, H., Lee, S. & Choi, C. H. Overestimation of photoelectrochemical hydrogen evolution reactivity induced by noble metal impurities dissolved from counter/reference electrodes. ACS Catal. 10 , 3381–3389 (2020).
Jerkiewicz, G. Applicability of platinum as a counter-electrode material in electrocatalysis research. ACS Catal. 12 , 2661–2670 (2022).
Guo, J., Hsu, A., Chu, D. & Chen, R. Improving oxygen reduction reaction activities on carbon-supported ag nanoparticles in alkaline solutions. J. Phys. Chem. C. 114 , 4324–4330 (2010).
Arulmozhi, N., Esau, D., van Drunen, J. & Jerkiewicz, G. Design and development of instrumentations for the preparation of platinum single crystals for electrochemistry and electrocatalysis research Part 3: Final treatment, electrochemical measurements, and recommended laboratory practices. Electrocatal 9 , 113–123 (2017).
Colburn, A. W., Levey, K. J., O’Hare, D. & Macpherson, J. V. Lifting the lid on the potentiostat: a beginner’s guide to understanding electrochemical circuitry and practical operation. Phys. Chem. Chem. Phys. 23 , 8100–8117 (2021).
Ban, Z., Kätelhön, E. & Compton, R. G. Voltammetry of porous layers: staircase vs analog voltammetry. J. Electroanal. Chem. 776 , 25–33 (2016).
McMath, A. A., Van Drunen, J., Kim, J. & Jerkiewicz, G. Identification and analysis of electrochemical instrumentation limitations through the study of platinum surface oxide formation and reduction. Anal. Chem. 88 , 3136–3143 (2016).
Jerkiewicz, G. Standard and reversible hydrogen electrodes: theory, design, operation, and applications. ACS Catal. 10 , 8409–8417 (2020).
Ives, D. J. G. & Janz, G. J. Reference Electrodes, Theory and Practice (Academic Press, 1961).
Newman, J. & Balsara, N. P. Electrochemical Systems (Wiley, 2021).
Inzelt, G., Lewenstam, A. & Scholz, F. Handbook of Reference Electrodes (Springer Berlin, 2013).
Cimenti, M., Co, A. C., Birss, V. I. & Hill, J. M. Distortions in electrochemical impedance spectroscopy measurements using 3-electrode methods in SOFC. I—effect of cell geometry. Fuel Cells 7 , 364–376 (2007).
Hoshi, Y. et al. Optimization of reference electrode position in a three-electrode cell for impedance measurements in lithium-ion rechargeable battery by finite element method. J. Power Sources 288 , 168–175 (2015).
Article ADS CAS Google Scholar
Jackson, C., Lin, X., Levecque, P. B. J. & Kucernak, A. R. J. Toward understanding the utilization of oxygen reduction electrocatalysts under high mass transport conditions and high overpotentials. ACS Catal. 12 , 200–211 (2022).
Masa, J., Batchelor-McAuley, C., Schuhmann, W. & Compton, R. G. Koutecky-Levich analysis applied to nanoparticle modified rotating disk electrodes: electrocatalysis or misinterpretation. Nano Res. 7 , 71–78 (2014).
Martens, S. et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources 392 , 274–284 (2018).
Wei, C. et al. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 48 , 2518–2534 (2019).
Lukaszewski, M., Soszko, M. & Czerwiński, A. Electrochemical methods of real surface area determination of noble metal electrodes—an overview. Int. J. Electrochem. Sci. https://doi.org/10.20964/2016.06.71 (2016).
Niu, S., Li, S., Du, Y., Han, X. & Xu, P. How to reliably report the overpotential of an electrocatalyst. ACS Energy Lett. 5 , 1083–1087 (2020).
Ohno, S. et al. How certain are the reported ionic conductivities of thiophosphate-based solid electrolytes? An interlaboratory study. ACS Energy Lett. 5 , 910–915 (2020).
Buck, R. P. et al. Measurement of pH. Definition, standards, and procedures (IUPAC Recommendations 2002). Pure Appl. Chem. https://doi.org/10.1351/pac200274112169 (2003).
Bell, S. Good Practice Guide No. 11. The Beginner’s Guide to Uncertainty of Measurement. (Issue 2). (National Physical Laboratory, 2001).
United Kingdom Accreditation Service. M3003 The Expression of Uncertainty and Confidence in Measurement 4th edn. (United Kingdom Accreditation Service, 2019).
Download references
Acknowledgements
This work was supported by the National Measurement System of the UK Department of Business, Energy & Industrial Strategy. Andy Wain, Richard Brown and Gareth Hinds (National Physical Laboratory, Teddington, UK) provided insightful comments on the text.
Author information
Authors and affiliations.
National Physical Laboratory, Hampton Road, Teddington, TW11 0LW, UK
Graham Smith & Edmund J. F. Dickinson
You can also search for this author in PubMed Google Scholar
Contributions
G.S. originated the concept for the article. G.S. and E.J.F.D. contributed to drafting, editing and revision of the manuscript.
Corresponding author
Correspondence to Graham Smith .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Gregory Jerkiewicz for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Smith, G., Dickinson, E.J.F. Error, reproducibility and uncertainty in experiments for electrochemical energy technologies. Nat Commun 13 , 6832 (2022). https://doi.org/10.1038/s41467-022-34594-x
Download citation
Received : 29 July 2022
Accepted : 31 October 2022
Published : 11 November 2022
DOI : https://doi.org/10.1038/s41467-022-34594-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Electrochemical surface-enhanced raman spectroscopy.
- Christa L. Brosseau
- Alvaro Colina
Nature Reviews Methods Primers (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Error, reproducibility and uncertainty in experiments for electrochemical energy technologies
Graham smith, edmund j f dickinson.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2022 Jul 29; Accepted 2022 Oct 31; Collection date 2022.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
The authors provide a metrology-led perspective on best practice for the electrochemical characterisation of materials for electrochemical energy technologies. Such electrochemical experiments are highly sensitive, and their results are, in practice, often of uncertain quality and challenging to reproduce quantitatively.
Subject terms: Electrocatalysis, Materials for energy and catalysis, Electrochemistry, Fuel cells, Batteries
A critical aspect of research on electrochemical energy devices, such as batteries, fuel cells and electrolysers, is the evaluation of new materials, components, or processes in electrochemical cells, either ex situ, in situ or in operation. For such experiments, rigorous experimental control and standardised methods are required to achieve reproducibility, even on standard or idealised systems such as single crystal platinum 1 . Data reported for novel materials often exhibit high (or unstated) uncertainty and often prove challenging to reproduce quantitatively. This situation is exacerbated by a lack of formally standardised methods, and practitioners with less formal training in electrochemistry being unaware of best practices. This limits trust in published metrics, with discussions on novel electrochemical systems frequently focusing on a single series of experiments performed by one researcher in one laboratory, comparing the relative performance of the novel material against a claimed state-of-the-art.
Much has been written about the broader reproducibility/replication crisis 2 and those reading the electrochemical literature will be familiar with weakly underpinned claims of “outstanding” performance, while being aware that comparisons may be invalidated by measurement errors introduced by experimental procedures which violate best practice; such issues frequently mar otherwise exciting science in this area. The degree of concern over the quality of reported results is evidenced by the recent decision of several journals to publish explicit experimental best practices 3 – 5 , reporting guidelines or checklists 6 – 10 and commentary 11 – 13 aiming to improve the situation, including for parallel theoretical work 14 .
We write as two electrochemists who, working in a national metrology institute, have enjoyed recent exposure to metrology: the science of measurement. Metrology provides the vocabulary 15 and mathematical tools 16 to express confidence in measurements and the comparisons made between them. Metrological systems and frameworks for quantification underpin consistency and assurance in all measurement fields and formal metrology is an everyday consideration for practical and academic work in fields where accurate measurements are crucial; we have found it a useful framework within which to evaluate our own electrochemical work. Here, rather than pen another best practice guide, we aim, with focus on three-electrode electrochemical measurements for energy material characterisation, to summarise some advice that we hope helps those performing electrochemical experiments to:
avoid mistakes and minimise error
report in a manner that facilitates reproducibility
consider and quantify uncertainty
Minimising mistakes and error
Metrology dispenses with nebulous concepts such as performance and instead requires scientists to define a specific measurand (“the quantity intended to be measured”) along with a measurement model ( ”the mathematical relation among all quantities known to be involved in a measurement”), which converts the experimental indicators into the measurand 15 . Error is the difference between the reported value of this measurand and its unknowable true value. (Note this is not the formal definition, and the formal concepts of error and true value are not fully compatible with measurement concepts discussed in this article, but we retain it here—as is common in metrology tuition delivered by national metrology institutes—for pedagogical purposes 15 ).
Mistakes (or gross errors) are those things which prevent measurements from working as intended. In electrochemistry the primary experimental indicator is often current or voltage, while the measurand might be something simple, like device voltage for a given current density, or more complex, like a catalyst’s turnover frequency. Both of these are examples of ‘method-defined measurands’, where the results need to be defined in reference to the method of measurement 17 , 18 (for example, to take account of operating conditions). Robust experimental design and execution are vital to understand, quantify and minimise sources of error, and to prevent mistakes.
Contemporary electrochemical instrumentation can routinely offer a current resolution and accuracy on the order of femtoamps; however, one electron looks much like another to a potentiostat. Consequently, the practical limit on measurements of current is the scientist’s ability to unambiguously determine what causes the observed current. Crucially, they must exclude interfering processes such as modified/poisoned catalyst sites or competing reactions due to impurities.
As electrolytes are conventionally in enormous excess compared to the active heterogeneous interface, electrolyte purity requirements are very high. Note, for example, that a perfectly smooth 1 cm 2 polycrystalline platinum electrode has on the order of 2 nmol of atoms exposed to the electrolyte, so that irreversibly adsorbing impurities present at the part per billion level (nmol mol −1 ) in the electrolyte may substantially alter the surface of the electrode. Sources of impurities at such low concentration are innumerable and must be carefully considered for each experiment; impurity origins for kinetic studies in aqueous solution have been considered broadly in the historical literature, alongside a review of standard mitigation methods 19 . Most commercial electrolytes contain impurities and the specific ‘grade’ chosen may have a large effect; for example, one study showed a three-fold decrease in the specific activity of oxygen reduction catalysts when preparing electrolytes with American Chemical Society (ACS) grade acid rather than a higher purity grade 20 . Likewise, even 99.999% pure hydrogen gas, frequently used for sparging, may contain more than the 0.2 μmol mol −1 of carbon monoxide permitted for fuel cell use 21 .
The most insidious impurities are those generated in situ. The use of reference electrodes with chloride-containing filling solutions should be avoided where chloride may poison catalysts 22 or accelerate dissolution. Similarly, reactions at the counter electrode, including dissolution of the electrode itself, may result in impurities. This is sometimes overlooked when platinum counter electrodes are used to assess ‘platinum-free’ electrocatalysts, accidentally resulting in performance-enhancing contamination 23 , 24 ; a critical discussion on this topic has recently been published 25 . Other trace impurity sources include plasticisers present in cells and gaskets, or silicates from the inappropriate use of glass when working with alkaline electrolytes 26 . To mitigate sensitivity to impurities from the environment, cleaning protocols for cells and components must be robust 27 . The use of piranha solution or similarly oxidising solution followed by boiling in Type 1 water is typical when performing aqueous electrochemistry 20 . Cleaned glassware and electrodes are also routinely stored underwater to prevent recontamination from airborne impurities.
The behaviour of electronic hardware used for electrochemical experiments should be understood and considered carefully in interpreting data 28 , recognising that the built-in complexity of commercially available digital potentiostats (otherwise advantageous!) is capable of introducing measurement artefacts or ambiguity 29 , 30 . While contemporary electrochemical instrumentation may have a voltage resolution of ~1 μV, its voltage measurement uncertainty is limited by other factors, and is typically on the order of 1 mV. As passing current through an electrode changes its potential, a dedicated reference electrode is often incorporated into both ex situ and, increasingly, in situ experiments to provide a stable well defined reference. Reference electrodes are typically selected from a range of well-known standardised electrode–electrolyte interfaces at which a characteristic and kinetically rapid reversible faradaic process occurs. The choice of reference electrode should be made carefully in consideration of chemical compatibility with the measurement environment 31 – 34 . In combination with an electronic blocking resistance, the potential of the electrode should be stable and reproducible. Unfortunately, deviation from the ideal behaviour frequently occurs. While this can often be overlooked when comparing results from identical cells, more attention is required when reporting values for comparison.
In all cases where conversion between different electrolyte–reference electrode systems is required, junction potentials should be considered. These arise whenever there are different chemical conditions in the electrolyte at the working electrode and reference electrode interfaces. Outside highly dilute solutions, or where there are large activity differences for a reactant/product of the electrode reaction (e.g. pH for hydrogen reactions), liquid junction potentials for conventional aqueous ions have been estimated in the range <50 mV 33 . Such a deviation may nonetheless be significant when onset potentials or activities at specific potentials are being reported. The measured potential difference between the working and reference electrode also depends strongly on the geometry of the cell, so cell design is critical. Fig. 1 shows the influence of cell design on potential profiles. Ideally the reference electrode should therefore be placed close to the working electrode (noting that macroscopic electrodes may have inhomogeneous potentials). To minimise shielding of the electric field between counter and working electrode and interruption of mass transport processes, a thin Luggin-Haber capillary is often used and a small separation maintained. Understanding of shielding and edge effects is vital when reference electrodes are introduced in situ. This is especially applicable for analysis of energy devices for which constraints on cell design, due to the need to minimise electrolyte resistance and seal the cell, preclude optimal reference electrode positioning 32 , 35 , 36 .
Fig. 1. Example of error introduced by cell geometry.
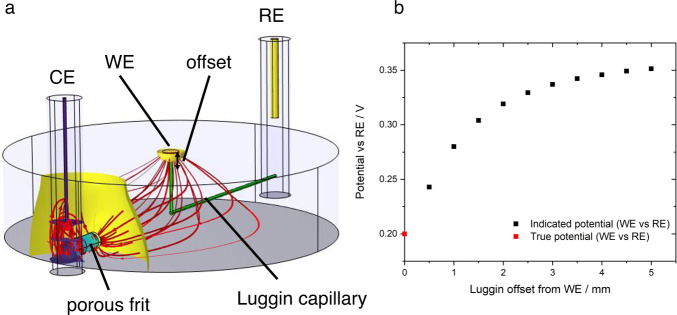
a Illustration (simulated data) of primary (resistive) current and potential distribution in a typical three-electrode cell. The main compartment is cylindrical (4 cm diameter, 1 cm height), filled with electrolyte with conductivity 1.28 S m −1 (0.1 M KCl(aq)). The working electrode (WE) is a 2 mm diameter disc drawing 1 mA (≈ 32 mA cm −2 ) from a faradaic process with infinitely fast kinetics and redox potential 0.2 V vs the reference electrode (RE). The counter electrode (CE) is connected to the main compartment by a porous frit; the RE is connected by a Luggin capillary (green cylinders) whose tip position is offset from the WE by a variable distance. Red lines indicate prevailing current paths; coloured surfaces indicate isopotential contours normal to the current density. b Plot of indicated WE vs RE potential (simulated data). As the Luggin tip is moved away from the WE surface, ohmic losses due to the WE-CE current distribution lead to variation in the indicated WE-RE potential. Appreciable error may arise on an offset length scale comparable to the WE radius.
Quantitative statements about fundamental electrochemical processes based on measured values of current and voltage inevitably rely on models of the system. Such models have assumptions that may be routinely overlooked when following experimental and analysis methods, and that may restrict their application to real-world systems. It is quite possible to make highly precise but meaningless measurements! An often-assumed condition for electrocatalyst analysis is the absence of mass transport limitation. For some reactions, such as the acidic hydrogen oxidation and hydrogen evolution reactions, this state is arguably so challenging to reach at representative conditions that it is impossible to measure true catalyst activity 11 . For example, ex situ thin-film rotating disk electrode measurements routinely fail to predict correct trends in catalyst performance in morphologically complex catalyst layers as relevant operating conditions (e.g. meaningful current densities) are theoretically inaccessible. This topic has been extensively discussed with some authors directly criticising this technique and exploring alternatives 37 , 38 , and others defending the technique’s applicability for ranking catalysts if scrupulous attention is paid to experimental details 39 ; yet, many reports continue to use this measurement technique blindly with no regard for its applicability. We therefore strongly urge those planning measurements to consider whether their chosen technique is capable of providing sufficient evidence to disprove their hypothesis, even if it has been widely used for similar experiments.
The correct choice of technique should be dependent upon the measurand being probed rather than simply following previous reports. The case of iR correction, where a measurement of the uncompensated resistance is used to correct the applied voltage, is a good example. When the measurand is a material property, such as intrinsic catalyst activity, the uncompensated resistance is a source of error introduced by the experimental method and it should carefully be corrected out (Fig. 1 ). In the case that the uncompensated resistance is intrinsic to the measurand—for instance the operating voltage of an electrolyser cell—iR compensation is inappropriate and only serves to obfuscate. Another example is the choice of ex situ (outside the operating environment), in situ (in the operating environment), and operando (during operation) measurements. While in situ or operando testing allows characterisation under conditions that are more representative of real-world use, it may also yield measurements with increased uncertainty due to the decreased possibility for fine experimental control. Depending on the intended use of the measurement, an informed compromise must be sought between how relevant and how uncertain the resulting measurement will be.
Maximising reproducibility
Most electrochemists assess the repeatability of measurements, performing the same measurement themselves several times. Repeats, where all steps (including sample preparation, where relevant) of a measurement are carried out multiple times, are absolutely crucial for highlighting one-off mistakes (Fig. 2 ). Reproducibility, however, is assessed when comparing results reported by different laboratories. Many readers will be familiar with the variability in key properties reported for various systems e.g. variability in the reported electrochemically active surface area (ECSA) of commercial catalysts, which might reasonably be expected to be constant, suggesting that, in practice, the reproducibility of results cannot be taken for granted. As electrochemistry deals mostly with method-defined measurands, the measurement procedure must be standardised for results to be comparable. Variation in results therefore strongly suggests that measurements are not being performed consistently and that the information typically supplied when publishing experimental methods is insufficient to facilitate reproducibility of electrochemical measurements. Quantitative electrochemical measurements require control over a large range of parameters, many of which are easily overlooked or specified imprecisely when reporting data. An understanding of the crucial parameters and methods for their control is often institutional knowledge, held by expert electrochemists, but infrequently formalised and communicated e.g. through publication of widely adopted standards. This creates challenges to both reproducibility and the corresponding assessment of experimental quality by reviewers. The reporting standards established by various publishers (see Introduction) offer a practical response, but it is still unclear whether these will contain sufficiently granular detail to improve the situation.
Fig. 2. Illustration of reproducibility, repeatability and gross error.
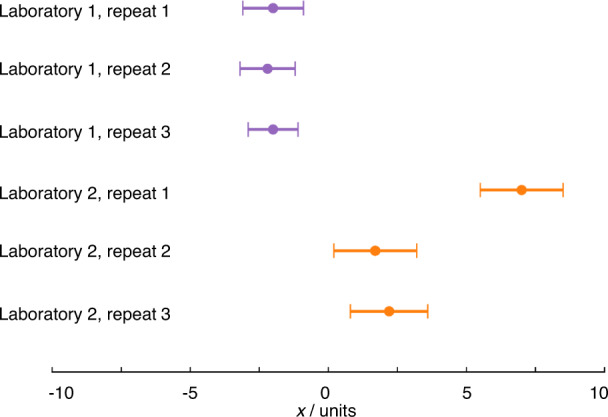
The measurements from laboratory 1 show a high degree of repeatability, while the measurements from laboratory 2 do not. Apparently, a mistake has been made in repeat 1, which will need to be excluded from any analysis and any uncertainty analysis, and/or suggests further repeat measurements should be conducted. The error bars are based on an uncertainty with coverage factor ~95% (see below) so the results from the two laboratories are different, i.e. show poor reproducibility. This may indicate differing experimental practice or that some as yet unidentified parameter is influencing the results.
Besides information typically supplied in the description of experimental methods for publication, which, at a minimum, must detail the materials, equipment and measurement methods used to generate the results, we suggest that a much more comprehensive description is often required, especially where measurements have historically poor reproducibility or the presented results differ from earlier reports. Such an expanded ‘supplementary experimental’ section would additionally include any details that could impact the results: for example, material pre-treatment, detailed electrode preparation steps, cleaning procedures, expected electrolyte and gas impurities, electrode preconditioning processes, cell geometry including electrode positions, detail of junctions between electrodes, and any other fine experimental details which might be institutional knowledge but unknown to the (now wide) readership of the electrochemical literature. In all cases any corrections and calculations used should be specified precisely and clearly justified; these may include determinations of properties of the studied system, such as ECSA, or of the environment, such as air pressure. We highlight that knowledge of the ECSA is crucial for conventional reporting of intrinsic electrocatalyst activity, but is often very challenging to measure in a reproducible manner 40 , 41 .
To aid reproducibility we recommend regularly calibrating experimental equipment and doing so in a way that is traceable to primary standards realising the International System of Units (SI) base units. The SI system ensures that measurement units (such as the volt) are uniform globally and invariant over time. Calibration applies to direct experimental indicators, e.g. loads and potentiostats, but equally to supporting tools such as temperature probes, balances, and flow meters. Calibration of reference electrodes is often overlooked even though variations from ideal behaviour can be significant 42 and, as discussed above, are often the limit of accuracy on potential measurement. Sometimes reports will specify internal calibration against a known reaction (such as the onset of the hydrogen evolution reaction), but rarely detail regular comparisons to a local master electrode artefact such as a reference hydrogen electrode or explain how that artefact is traceable, e.g. through control of the filling solution concentration and measurement conditions. If reference is made to a standardised material (e.g. commercial Pt/C) the specified material should be both widely available and the results obtained should be consistent with prior reports.
Beyond calibration and reporting, the best test of reproducibility is to perform intercomparisons between laboratories, either by comparing results to identical experiments reported in the literature or, more robustly, through participation in planned intercomparisons (for example ‘round-robin’ exercises); we highlight a recent study applied to solid electrolyte characterisation as a topical example 43 . Intercomparisons are excellent at establishing the key features of an experimental method and the comparability of results obtained from different methods; moreover they provide a consensus against which other laboratories may compare themselves. However, performing repeat measurements for assessing repeatability and reproducibility cannot estimate uncertainty comprehensively, as it excludes systematic sources of uncertainty.
Assessing measurement uncertainty
Formal uncertainty evaluation is an alien concept to most electrochemists; even the best papers (as well as our own!) typically report only the standard deviation between a few repeats. Metrological best practice dictates that reported values are stated as the combination of a best estimate of the measurand, and an interval, and a coverage factor ( k ) which gives the probability of the true value being within that interval. For example, “the turnover frequency of the electrocatalyst is 1.0 ± 0.2 s −1 ( k = 2)” 16 means that the scientist (having assumed normally distributed error) is 95% confident that the turnover frequency lies in the range 0.8–1.2 s −1 . Reporting results in such a way makes it immediately clear whether the measurements reliably support the stated conclusions, and enables meaningful comparisons between independent results even if their uncertainties differ (Fig. 3 ). It also encourages honesty and self-reflection about the shortcomings of results, encouraging the development of improved experimental techniques.
Fig. 3. Illustrating the role of uncertainty in deciding which result is higher.
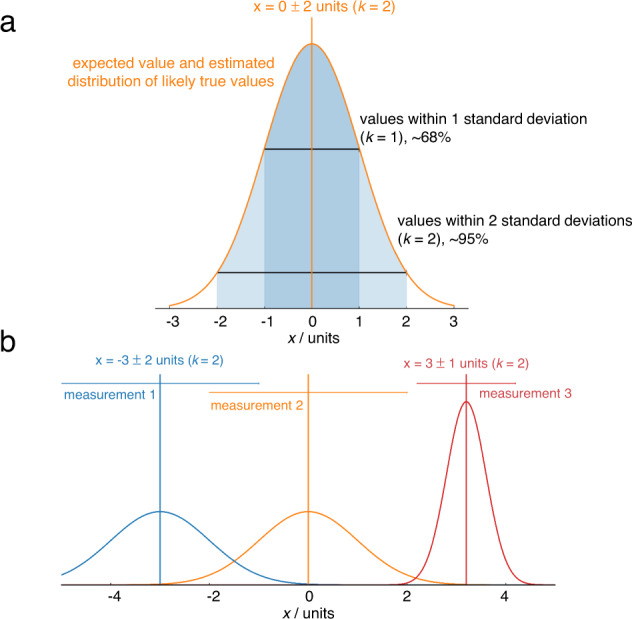
a Complete reporting of a measurement includes the best estimate of the measurand and an uncertainty and the probability the true value falls within the uncertainty reported. Here, the percentages indicate that a normal distribution has been assumed. b Horizontal bars indicate 95% confidence intervals from uncertainty analysis. The confidence intervals of measurements 1 and 2 overlap when using k = 2, so it is not possible to say with 95% confidence that the result of the measurement 2 is higher than measurement 1, but it is possible to say this with 68% confidence, i.e. k = 1. Measurement 3 has a lower uncertainty, so it is possible to say with 95% confidence that the value is higher than measurement 2.
Constructing such a statement and performing the underlying calculations often appears daunting, not least as there are very few examples for electrochemical systems, with pH measurements being one example to have been treated thoroughly 44 . However, a standard process for uncertainty analysis exists, as briefly outlined graphically in Fig. 4 . We refer the interested reader to both accessible introductory texts 45 and detailed step-by-step guides 16 , 46 . The first steps in the process are to state precisely what is being measured—the measurand—and identify likely sources of uncertainty. Even this qualitative effort is often revealing. Precision in the definition of the measurand (and how it is determined from experimental indicators) clarifies the selection of measurement technique and helps to assess its appropriateness; for example, where the measurand relates only to an instantaneous property of a specific physical object, e.g. the current density of a specific fuel cell at 0.65 V following a standardised protocol, we ignore all variability in construction, device history etc. and no error is introduced by the sample. Whereas, when the measurand is a material property, such as turnover frequency of a catalyst material with a defined chemistry and preparation method, variability related to the material itself and sample preparation will often introduce substantial uncertainty in the final result. In electrochemical measurements, errors may arise from a range of sources including the measurement equipment, fluctuations in operating conditions, or variability in materials and samples. Identifying these sources leads to the design of better-quality experiments. In essence, the subsequent steps in the calculation of uncertainty quantify the uncertainty introduced by each source of error and, by using a measurement model or a sensitivity analysis (i.e. an assessment of how the results are sensitive to variability in input parameters), propagate these to arrive at a final uncertainty on the reported result.
Fig. 4. Illustration of simplified and exaggerated uncertainty evaluation on open circuit potential vs a reference electrode (RE).
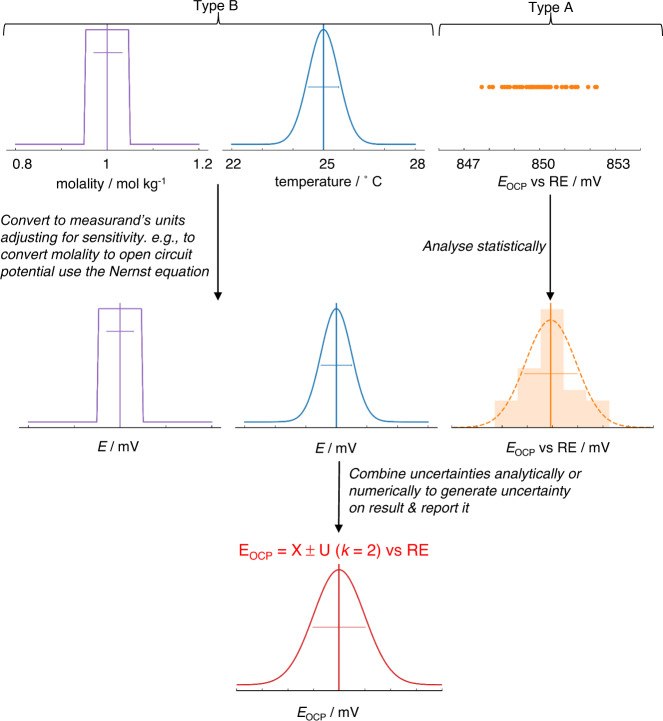
Possible sources of uncertainty are identified, and their standard uncertainty or probability distribution is determined by statistical analysis of repeat measurements (Type A uncertainties) or other evidence (Type B uncertainties). If required, uncertainties are then converted into the same unit as the measurand and adjusted for sensitivity, using a measurement model. Uncertainties are then combined either analytically using a standard approach or numerically to generate an overall estimate of uncertainty for the measurand (as indicated in Fig. 3a ).
Generally, given the historically poor understanding of uncertainty in electrochemistry, we promote increased awareness of uncertainty reporting standards and a focus on reporting measurement uncertainty with a level of detail that is appropriate to the claim made, or the scientific utilisation of the data. For example, where the primary conclusion of a paper relies on demonstrating that a material has the ‘highest ever X’ or ‘X is bigger than Y’ it is reasonable for reviewers to ask authors to quantify how confident they are in their measurement and statement. Additionally, where uncertainties are reported, even with error bars in numerical or graphical data, the method by which the uncertainty was determined should be stated, even if the method is consciously simple (e.g. “error bars indicate the sample standard deviation of n = 3 measurements carried out on independent electrodes”). Unfortunately, we are aware of only sporadic and incomplete efforts to create formal uncertainty budgets for electrochemical measurements of energy technologies or materials, though work is underway in our group to construct these for some exemplar systems.
Electrochemistry has undoubtedly thrived without significant interaction with formal metrology; we do not urge an abrupt revolution whereby rigorous measurements become devalued if they lack additional arcane formality. Rather, we recommend using the well-honed principles of metrology to illuminate best practice and increase transparency about the strengths and shortcomings of reported experiments. From rethinking experimental design, to participating in laboratory intercomparisons and estimating the uncertainty on key results, the application of metrological principles to electrochemistry will result in more robust science.
Acknowledgements
This work was supported by the National Measurement System of the UK Department of Business, Energy & Industrial Strategy. Andy Wain, Richard Brown and Gareth Hinds (National Physical Laboratory, Teddington, UK) provided insightful comments on the text.
Author contributions
G.S. originated the concept for the article. G.S. and E.J.F.D. contributed to drafting, editing and revision of the manuscript.
Peer review
Peer review information.
Nature Communications thanks Gregory Jerkiewicz for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Climent, V. & Feliu, J. M. Thirty years of platinum single crystal electrochemistry. J. Solid State Electrochem . 10.1007/s10008-011-1372-1 (2011).
- 2. Nature Editors and Contributors. Challenges in irreproducible research collection. Nature https://www.nature.com/collections/prbfkwmwvz/ (2018).
- 3. Chen JG, Jones CW, Linic S, Stamenkovic VR. Best practices in pursuit of topics in heterogeneous electrocatalysis. ACS Catal. 2017;7:6392–6393. doi: 10.1021/acscatal.7b02839. [ DOI ] [ Google Scholar ]
- 4. Voiry D, et al. Best practices for reporting electrocatalytic performance of nanomaterials. ACS Nano. 2018;12:9635–9638. doi: 10.1021/acsnano.8b07700. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Wei C, et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 2019;31:1806296. doi: 10.1002/adma.201806296. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Chem Catalysis Editorial Team. Chem Catalysis Checklists Revision 1.1 . https://info.cell.com/introducing-our-checklists-learn-more (2021).
- 7. Chatenet M, et al. Good practice guide for papers on fuel cells and electrolysis cells for the Journal of Power Sources. J. Power Sources. 2020;451:227635. doi: 10.1016/j.jpowsour.2019.227635. [ DOI ] [ Google Scholar ]
- 8. Sun YK. An experimental checklist for reporting battery performances. ACS Energy Lett. 2021;6:2187–2189. doi: 10.1021/acsenergylett.1c00870. [ DOI ] [ Google Scholar ]
- 9. Li J, et al. Good practice guide for papers on batteries for the Journal of Power Sources. J. Power Sources. 2020;452:227824. doi: 10.1016/j.jpowsour.2020.227824. [ DOI ] [ Google Scholar ]
- 10. Arbizzani C, et al. Good practice guide for papers on supercapacitors and related hybrid capacitors for the Journal of Power Sources. J. Power Sources. 2020;450:227636. doi: 10.1016/j.jpowsour.2019.227636. [ DOI ] [ Google Scholar ]
- 11. Hansen JN, et al. Is there anything better than Pt for HER? ACS Energy Lett. 2021;6:1175–1180. doi: 10.1021/acsenergylett.1c00246. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Xu K. Navigating the minefield of battery literature. Commun. Mater. 2022;3:1–7. doi: 10.1038/s43246-022-00251-5. [ DOI ] [ Google Scholar ]
- 13. Dix ST, Lu S, Linic S. Critical practices in rigorously assessing the inherent activity of nanoparticle electrocatalysts. ACS Catal. 2020;10:10735–10741. doi: 10.1021/acscatal.0c03028. [ DOI ] [ Google Scholar ]
- 14. Mistry A, et al. A minimal information set to enable verifiable theoretical battery research. ACS Energy Lett. 2021;6:3831–3835. doi: 10.1021/acsenergylett.1c01710. [ DOI ] [ Google Scholar ]
- 15. Joint Committee for Guides in Metrology: Working Group 2. International Vocabulary of Metrology—Basic and General Concepts and Associated Terms . (2012).
- 16. Joint Committee for Guides in Metrology: Working Group 1. Evaluation of measurement data—Guide to the expression of uncertainty in measurement . (2008).
- 17. International Organization for Standardization: Committee on Conformity Assessment. ISO 17034:2016 General requirements for the competence of reference material producers . (2016).
- 18. Brown, R. J. C. & Andres, H. How should metrology bodies treat method-defined measurands? Accredit. Qual. Assur . 10.1007/s00769-020-01424-w (2020).
- 19. Angerstein-Kozlowska, H. Surfaces, Cells, and Solutions for Kinetics Studies . Comprehensive Treatise of Electrochemistry vol. 9: Electrodics: Experimental Techniques (Plenum Press, 1984).
- 20. Shinozaki K, Zack JW, Richards RM, Pivovar BS, Kocha SS. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique. J. Electrochem. Soc. 2015;162:F1144–F1158. doi: 10.1149/2.1071509jes. [ DOI ] [ Google Scholar ]
- 21. International Organization for Standardization: Technical Committee ISO/TC 197. ISO 14687:2019(E)—Hydrogen fuel quality—Product specification . (2019).
- 22. Schmidt TJ, Paulus UA, Gasteiger HA, Behm RJ. The oxygen reduction reaction on a Pt/carbon fuel cell catalyst in the presence of chloride anions. J. Electroanal. Chem. 2001;508:41–47. doi: 10.1016/S0022-0728(01)00499-5. [ DOI ] [ Google Scholar ]
- 23. Chen R, et al. Use of platinum as the counter electrode to study the activity of nonprecious metal catalysts for the hydrogen evolution reaction. ACS Energy Lett. 2017;2:1070–1075. doi: 10.1021/acsenergylett.7b00219. [ DOI ] [ Google Scholar ]
- 24. Ji SG, Kim H, Choi H, Lee S, Choi CH. Overestimation of photoelectrochemical hydrogen evolution reactivity induced by noble metal impurities dissolved from counter/reference electrodes. ACS Catal. 2020;10:3381–3389. doi: 10.1021/acscatal.9b04229. [ DOI ] [ Google Scholar ]
- 25. Jerkiewicz G. Applicability of platinum as a counter-electrode material in electrocatalysis research. ACS Catal. 2022;12:2661–2670. doi: 10.1021/acscatal.1c06040. [ DOI ] [ Google Scholar ]
- 26. Guo J, Hsu A, Chu D, Chen R. Improving oxygen reduction reaction activities on carbon-supported ag nanoparticles in alkaline solutions. J. Phys. Chem. C. 2010;114:4324–4330. doi: 10.1021/jp910790u. [ DOI ] [ Google Scholar ]
- 27. Arulmozhi N, Esau D, van Drunen J, Jerkiewicz G. Design and development of instrumentations for the preparation of platinum single crystals for electrochemistry and electrocatalysis research Part 3: Final treatment, electrochemical measurements, and recommended laboratory practices. Electrocatal. 2017;9:113–123. doi: 10.1007/s12678-017-0426-2. [ DOI ] [ Google Scholar ]
- 28. Colburn AW, Levey KJ, O’Hare D, Macpherson JV. Lifting the lid on the potentiostat: a beginner’s guide to understanding electrochemical circuitry and practical operation. Phys. Chem. Chem. Phys. 2021;23:8100–8117. doi: 10.1039/D1CP00661D. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Ban Z, Kätelhön E, Compton RG. Voltammetry of porous layers: staircase vs analog voltammetry. J. Electroanal. Chem. 2016;776:25–33. doi: 10.1016/j.jelechem.2016.06.003. [ DOI ] [ Google Scholar ]
- 30. McMath AA, Van Drunen J, Kim J, Jerkiewicz G. Identification and analysis of electrochemical instrumentation limitations through the study of platinum surface oxide formation and reduction. Anal. Chem. 2016;88:3136–3143. doi: 10.1021/acs.analchem.5b04239. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Jerkiewicz G. Standard and reversible hydrogen electrodes: theory, design, operation, and applications. ACS Catal. 2020;10:8409–8417. doi: 10.1021/acscatal.0c02046. [ DOI ] [ Google Scholar ]
- 32. Ives, D. J. G. & Janz, G. J. Reference Electrodes, Theory and Practice (Academic Press, 1961).
- 33. Newman, J. & Balsara, N. P. Electrochemical Systems (Wiley, 2021).
- 34. Inzelt, G., Lewenstam, A. & Scholz, F. Handbook of Reference Electrodes (Springer Berlin, 2013).
- 35. Cimenti M, Co AC, Birss VI, Hill JM. Distortions in electrochemical impedance spectroscopy measurements using 3-electrode methods in SOFC. I—effect of cell geometry. Fuel Cells. 2007;7:364–376. doi: 10.1002/fuce.200700019. [ DOI ] [ Google Scholar ]
- 36. Hoshi Y, et al. Optimization of reference electrode position in a three-electrode cell for impedance measurements in lithium-ion rechargeable battery by finite element method. J. Power Sources. 2015;288:168–175. doi: 10.1016/j.jpowsour.2015.04.065. [ DOI ] [ Google Scholar ]
- 37. Jackson C, Lin X, Levecque PBJ, Kucernak ARJ. Toward understanding the utilization of oxygen reduction electrocatalysts under high mass transport conditions and high overpotentials. ACS Catal. 2022;12:200–211. doi: 10.1021/acscatal.1c03908. [ DOI ] [ Google Scholar ]
- 38. Masa J, Batchelor-McAuley C, Schuhmann W, Compton RG. Koutecky-Levich analysis applied to nanoparticle modified rotating disk electrodes: electrocatalysis or misinterpretation. Nano Res. 2014;7:71–78. doi: 10.1007/s12274-013-0372-0. [ DOI ] [ Google Scholar ]
- 39. Martens S, et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources. 2018;392:274–284. doi: 10.1016/j.jpowsour.2018.04.084. [ DOI ] [ Google Scholar ]
- 40. Wei C, et al. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019;48:2518–2534. doi: 10.1039/C8CS00848E. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Lukaszewski, M., Soszko, M. & Czerwiński, A. Electrochemical methods of real surface area determination of noble metal electrodes—an overview. Int. J. Electrochem. Sci. 10.20964/2016.06.71 (2016).
- 42. Niu S, Li S, Du Y, Han X, Xu P. How to reliably report the overpotential of an electrocatalyst. ACS Energy Lett. 2020;5:1083–1087. doi: 10.1021/acsenergylett.0c00321. [ DOI ] [ Google Scholar ]
- 43. Ohno S, et al. How certain are the reported ionic conductivities of thiophosphate-based solid electrolytes? An interlaboratory study. ACS Energy Lett. 2020;5:910–915. doi: 10.1021/acsenergylett.9b02764. [ DOI ] [ Google Scholar ]
- 44. Buck, R. P. et al. Measurement of pH. Definition, standards, and procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 10.1351/pac200274112169 (2003).
- 45. Bell, S. Good Practice Guide No. 11. The Beginner’s Guide to Uncertainty of Measurement. (Issue 2). (National Physical Laboratory, 2001).
- 46. United Kingdom Accreditation Service. M3003 The Expression of Uncertainty and Confidence in Measurement 4th edn. (United Kingdom Accreditation Service, 2019).
- View on publisher site
- PDF (782.9 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Uncertainty, Accuracy, and Precision
Sources of Uncertainty in Measurements in the Lab
When taking a measurement or performing an experiment, there are many ways in which uncertainty can appear, even if the procedure is performed exactly as indicated. Each experiment and measurement needs to be considered carefully to identify potential limitations or tricky procedural spots.
When considering sources of error for a lab report be sure to consult with your lab manual and/or TA , as each course has different expectations on what types of error or uncertainty sources are expected to be discussed.
Types of Uncertainty or Error
While these are not sources of error, knowing the two main ways we classify uncertainty or error in a measurement may help you when considering your own experiments.
Systematic Error
Systematic errors are those that affect the accuracy of your final value. These can often be greatly reduced or eliminated entirely by adjusting your procedure. These errors usually exist and are often constant for the duration of the experiment – or if changing slightly, like an instrument reading “drifting” with time, they are in a consistent direction (higher or lower than the “true” value).
One example of a systematic error could be using a pH meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a pH 6.00 buffer. Another could be doing calculations using an equilibrium constant derived for a temperature of 25.0° C when the experiment was done at 20.0° C.
Random Error
Random errors are those that primarily affect the precision of your final value. Random error can usually be reduced by adjusting the procedure or increasing skill of the experimenter, but can never be completely eliminated.
You can observe random error when you weigh an object (say, recording a mass of 1.0254 g) and when re-weighing it, you get a slightly different measurement (say 1.0255 g). Another example is the interpolation of the final digit on a scale, as in the example earlier in this section . In a group of people observing the same meniscus you expect to get a range of readings, mostly between 25.5 – 25.7 mL.
Some Common Sources of Error
Every experiment is different, but if you are analyzing your procedure for potential sources of uncertainty, there are a few places you can start:
Assumptions About Physical Status
Every procedure comes with some assumptions. Perhaps you assume that the room temperature is 25.0° C (most UCalgary building HVAC is set to 21 ° C and fluctuates around that). Maybe you assumed a typical ambient air pressure without taking a measurement of the actual value. Perhaps you further assume that physical constants, like equilibrium constants, enthalpies, and others, do not change (much) from 25.0° C to the actual ambient temperature. Perhaps you used a “literature value” rather than measuring that quantity under your own experimental conditions.
You may have also made assumptions about your reaction – that it went to completion, or that you were able to detect a colour change visually that indicated completion (but may have really been 60, 80, or 90% complete). Perhaps there is a “side reaction” that can happen, or your product was not purified or dried thoroughly in this procedure.
There are many places where assumptions (appropriate or not) appear: depending on the difference between assumed and real conditions, this may add a negligible amount of uncertainty, or even a few percent, depending on the measurement.

Limitations on Measurements
As we have seen throughout this section, every measurement has a limit – often expressed through its recorded precision or significant figures. Some equipment can be used more precisely than others: for example, a Mohr (or serological) pipet can at best be used to $\pm$ 0.1 mL precision, while a transfer (or analytical) pipet may be used to $\pm$ 0.01 mL precision.
The less-precise equipment is usually easier and faster to use, but when precision is important, be sure you have chosen the appropriate glassware or equipment for your measurement.
Limitations on Calculations
Generally, laboratory calculations reflect the precision of a measurement, rather than limiting it (or directly affecting the accuracy). However some particular points can be sources of uncertainty.
Use of physical constants can limit your accuracy or precision if you use a rounded version (e.g. $3.00\times 10^{8}$ m/s instead of 299 792 458 m/s. As discussed above, using a value that is determined for a different physical state (temperature, pressure, etc) may also introduce some error.
Creating and reading graphs can be a major source of uncertainty if done sloppily. Remember you can only read your graph as precisely as your gridlines allow : most people can accurately interpolate to 1/10 of a division at best. You may also (manually or by regression) plot a line of best fit: this line is only as good as your data, and your calculations based on it may be limited by the precision at which you have drawn or calculated this line. The video below gives some starting tips for using Excel to create a graph appropriate for a first-year chemistry laboratory report.
“To err is human” … but not all such human error is acceptable in a procedure. Some limitations are unavoidable: for example a colourblind person reading pH from a colour indicator, or a time-dependent procedure step that is tricky to complete quickly and accurately. Often, these can be designed out of a procedure, or corrected by repeating the measurement.
True mistakes along the lines of “I overfilled the volumetric flask” should be corrected in the lab if at all possible. This may be (for example) re-making the solution, or measuring the overfill to determine the true volume used in the flask. There is usually no excuse for allowing a mistake to remain in your experiment , especially if there was time to correct or repeat the measurement. If a mistake happened and you could not correct it, you should include that in your lab report – but know that it may not be enough for a complete “sources of error” discussion.
Learning Materials
- Business Studies
- Combined Science
- Computer Science
- Engineering
- English Literature
- Environmental Science
- Human Geography
- Macroeconomics
- Microeconomics
- Sources of Error in Experiments
Discover the intricacies and implications of sources of error in experiments, specifically in the context of engineering. This comprehensive guide elaborates on what these errors mean, how they influence results, and strategies to mitigate their impact. Dive deep into the world of experimental errors, with detailed exploration of common examples, their potential consequences and effective solutions. Whether you're undertaking your first engineering experiment or a seasoned professional, understanding these errors is crucial to ensuring your data remains valid, reliable and accurate. Let's embark on this educational journey, delving into the art of identifying and managing sources of error in experiments.
Millions of flashcards designed to help you ace your studies
- Cell Biology
What is the meaning of 'error' in the context of an experiment?
What are some strategies to minimise sources of error in engineering experiments?
Why is conducting a pre-experimental design analysis significant in reducing errors in engineering experiments?
What is a parallax error in an experiment?
What are systematic errors and how do they impact experiments?
What are the three primary sources of errors in engineering experiments?
How do the sources of errors affect engineering experiments and why is understanding them crucial?
What could cause instrumental drift in engineering experiments?
What does a sample contamination error indicate?
What is a random error and how does it affect an experiment?
What is the consequence of blunders in an experiment?
Need help? Meet our AI Assistant

Need help with Sources of Error in Experiments? Ask our AI Assistant

Review generated flashcards
to start learning or create your own AI flashcards
Start learning or create your own AI flashcards

Vaia Editorial Team
Team Sources of Error in Experiments Teachers
- 19 minutes reading time
- Checked by Vaia Editorial Team
- Aerospace Engineering
- Artificial Intelligence & Engineering
- Automotive Engineering
- Chemical Engineering
- Design Engineering
- Engineering Fluid Mechanics
- Engineering Mathematics
- Engineering Thermodynamics
- Materials Engineering
- Mechanical Engineering
- Professional Engineering
- Accreditation
- Activity Based Costing
- Array in Excel
- Balanced Scorecard
- Business Excellence Model
- Calibration
- Conditional Formatting
- Consumer Protection Act 1987
- Continuous Improvement
- Data Analysis in Engineering
- Data Management
- Data Visualization
- Design of Engineering Experiments
- Diversity and Inclusion
- Elements of Cost
- Embodied Energy
- End of Life Product
- Engineering Institution
- Engineering Law
- Engineering Literature Review
- Engineering Organisations
- Engineering Skills
- Environmental Management System
- Environmental Protection Act 1990
- Error Analysis
- Excel Charts
- Excel Errors
- Excel Formulas
- Excel Operators
- Finance in Engineering
- Financial Management
- Formal Organizational Structure
- Health & Safety at Work Act 1974
- Henry Mintzberg
- IF Function Excel
- INDEX MATCH Excel
- IP Licensing
- ISO 9001 Quality Manual
- Initial Public Offering
- Intellectual Property
- MAX Function Excel
- Machine Guarding
- McKinsey 7S Framework
- Measurement Techniques
- National Measurement Institute
- Network Diagram
- Organizational Strategy Engineering
- Overhead Absorption
- Part Inspection
- Porter's Value Chain
- Professional Conduct
- Professional Development
- Profit and Loss
- Project Control
- Project Life Cycle
- Project Management
- Project Risk Management
- Project Team
- Quality Tools
- Resource Constrained Project Scheduling
- Risk Analysis
- Risk Assessment
- Root Cause Analysis
- Sale of Goods Act 1979
- Situational Factors
- Six Sigma Methodology
- Standard Cost
- Statistical Process Control
- Strategic Management
- Supply Chain Engineering
- Surface Texture Measurement
- Sustainable Engineering
- Sustainable Manufacturing
- Technical Presentation
- Technical Report
- Trade Secret vs Patent
- Venture Capital
- Viable System Model
- What is Microsoft Excel
- Work Breakdown Structure
- Robotics Engineering
- Solid Mechanics
- What is Engineering
Jump to a key chapter
Understanding the Meaning of Sources of Error in Experiments
In the context of an experiment, 'error' refers to the deviation of a measured or calculated value from the true value. The accumulation of these errors, if not properly addressed, can significantly impact the accuracy and validity of experimental results.
Explaining What Sources of Error in Experiments Mean in Engineering
- Systematic Errors
- Random Errors
'Systematic errors' are errors that are consistent and repeatable, and typically result from faulty equipment or a flawed experimental design.
If your scale is not properly zeroed and is instead always showing a measure 0.01 units above the actual mark, even if you measure a distance of 10 units perfectly, your recorded measure will be 10.01 units. This is a clear illustration of a systematic error.
The Role of These Sources of Error in Engineering Experiments
Here's an interesting aspect to consider: while errors in experiments are often seen as problematic, they sometimes lead to new discoveries. A classic example is the serendipitous discovery of penicillin by Alexander Fleming, resulting from a contamination (an ‘error’) in his experiment.
Common Examples of Sources of Error in Experiments
Classic instances of sources of error in experiments examples.
- Parallax error: This occurs when, due to line of sight issues, objects are wrongly positioned, usually resulting in overlap and causing the definite location of an object to be uncertain.
- Reading error: Taking the wrong value from the scale of the measuring instrument results in a reading error. This can be as straightforward as misreading the markings on a ruler or thermometer.
- Instrument precision error: Some instruments have limitations on how precisely they can measure. For instance, a typical school laboratory ruler can only measure to the nearest millimetre, while far more precise measurements may be required.
Parallax error, reading error, and instrument precision error all serve as practical examples of systematic errors. These errors are consistent and repeatable and are typically caused by the limitations of the experimental setup and equipment.
Frequently Encountered Types of Errors in Engineering Experiments
- Environmental fluctuations: Changes in environmental conditions such as temperature, humidity, pressure, or electromagnetic interference can all lead to errors in measurements.
- Instrumental drift: Many instruments display a change in response over time, leading to an error known as 'drift'. It's often seen in electronic components where properties can change due to warming up.
- Misuse of equipment: Incorrect use of equipment or using the wrong equipment for a specific measure can result in large errors in experimental data.
- Sample contamination: When carrying out chemical or biological experiments, contamination of samples can significantly alter results.
Consider a circuit with a nominal 10V supply that drifts by 0.1V over a one-hour experiment. If you are measuring a voltage drop over a 1kΩ resistor using Ohm’s law (\( V = IR \)), the potential error due to drift would be an incorrect calculation of current by 0.1mA. As measurements become more precise, this kind of drift can have a significant impact on results.
Diving into Sources of Error in Experiments and Their Impact
Sources of error in experiments and their potential consequences.
- For instance, consider a case where a faulty weighing scale always shows a measurable amount as 2 grams less than the actual. If you are using this scale to measure a chemical necessary for a series of chemical reactions, then irrespective of careful execution, the results will always be skewed due to this error.
- A classical example of a random error might occur in laboratory experiments involving the measurement of temperature. Day-to-day fluctuations in room temperature may cause slight variations in the measured temperature of a liquid, leading to inconsistent, and thus, unreliable results.
- An example might be if you inaccurately record the temperature as 800 degrees Celsius instead of 80 degrees Celsius. No matter how impeccably the rest of the experiment is performed, this blunder will translate into an error that could mask the real results.
How Errors in Experiments Affect Outcomes and Data Integrity
For simplicity, assume the reaction rate is linearly proportional to the amount of catalyst used, as described by the equation \[ R = kC \], where \( R \) is the reaction rate, \( k \) is the rate constant, and \( C \) is the concentration of the catalyst. If the scale you're using to measure the catalyst is off by the said 2 grams, this error will influence the value of \( k \) you determine from measurements. In effect, you’ve introduced an error in an experiment that was otherwise impeccably planned and executed.
Evaluating and Reducing Sources of Error in Engineering Experiments
Strategies for minimising sources of error in experiments.
Calibration is the process of sequentially adjusting the output or indication on a measurement instrument to match the values represented by a reference standard over the entire measurement range of the instrument.
Tools and Techniques to Keep Errors Minimal in Engineering Experiments
Analog devices represent data using a physical quantity that can take on any value within a range, while digital devices represent data as discrete values.
Solutions for Common Sources of Error in Experiments
Suggested problem-solving approaches for sources of error in experiments.
Systematic Errors: These occur due to predictable and consistent factors which cause the measured value to deviate from the true value. They result in a bias in the data.
Random Errors: These are unpredictable fluctuations that arise from variables in the experiment that are outside of control. Unlike systematic errors, they cannot be pinpointed to any specific factor and thus, add uncertainty to the experimental results.
Blunders: These are avoidable and usually arise due to misconceptions, carelessness or oversight. These are not inherent in the experimental procedure but entirely depend on human factors and thus, different from the first two types.
Methods to Counteract Frequent Sources of Error in Engineering Experiments
Sources of error in experiments - key takeaways.
- Sources of Error in Experiments can lead to valuable findings, as in the case of penicillin discovered by Alexander Fleming due to a 'contamination error'.
- Common errors in experiments include Parallax error, Reading error and Instrument precision error, all of which, if overlooked, can yield misleading results.
- Engineering experiments often encounter more specific errors such as Environmental fluctuations, Instrumental drift, Misuse of equipment, and Sample contamination.
- Systematic errors (consistent and repeatable), random errors (unpredictable and arising from variables outside an experimenter's control), and blunders (human errors resulting from carelessness or lack of knowledge) can significantly impact the accuracy of experimental results.
- Strategies for minimising Sources of Error include calibration of equipment, performing repetitions of the experiment, conducting a pre-experimental design analysis, error estimation, and carrying out blind trials.
Flashcards in Sources of Error in Experiments 15
In an experiment, 'error' refers to the deviation of a measured or calculated value from the true value. If not addressed, these can impact the accuracy and validity of experimental results.
Strategies include calibration of equipment, repeating trials to dilute random errors, preventing blunders through precise execution, pre-experimental design analysis, error estimation, and conducting blind trials to avoid bias.
This step involves identifying potential error sources beforehand and dealing with them in the experimental design phase, which helps in choosing the most appropriate equipment and minimising future errors.
A parallax error occurs when, due to line of sight issues, objects are wrongly positioned, usually resulting in overlap and causing the definite location of an object to be uncertain.
Systematic errors are repeatable inconsistencies, typically from faulty equipment or flawed experimental design. They consistently skew your results, affecting the reliability and accuracy of your conclusions.
The three primary sources of errors in engineering experiments are Systematic Errors, Random Errors, and Blunders.

Learn with 15 Sources of Error in Experiments flashcards in the free Vaia app
We have 14,000 flashcards about Dynamic Landscapes.
Already have an account? Log in
Frequently Asked Questions about Sources of Error in Experiments
Test your knowledge with multiple choice flashcards.
What is the meaning of 'error' in the context of an experiment?

Join the Vaia App and learn efficiently with millions of flashcards and more!
That was a fantastic start, you can do better, sign up to create your own flashcards.
Access over 700 million learning materials
Study more efficiently with flashcards
Get better grades with AI
Already have an account? Log in
Keep learning, you are doing great.
Discover learning materials with the free Vaia app

Vaia is a globally recognized educational technology company, offering a holistic learning platform designed for students of all ages and educational levels. Our platform provides learning support for a wide range of subjects, including STEM, Social Sciences, and Languages and also helps students to successfully master various tests and exams worldwide, such as GCSE, A Level, SAT, ACT, Abitur, and more. We offer an extensive library of learning materials, including interactive flashcards, comprehensive textbook solutions, and detailed explanations. The cutting-edge technology and tools we provide help students create their own learning materials. StudySmarter’s content is not only expert-verified but also regularly updated to ensure accuracy and relevance.
Team Engineering Teachers
Study anywhere. Anytime.Across all devices.
Create a free account to save this explanation..
Save explanations to your personalised space and access them anytime, anywhere!
By signing up, you agree to the Terms and Conditions and the Privacy Policy of Vaia.
Sign up to highlight and take notes. It’s 100% free.
Join over 22 million students in learning with our Vaia App
The first learning app that truly has everything you need to ace your exams in one place
- Flashcards & Quizzes
- AI Study Assistant
- Study Planner
- Smart Note-Taking

Privacy Overview
Monkey Physics Blog
For all things physics, how to write sources of error.

Sources of Error in Physics
This article will help you:
- learn how to identify sources of error for a physics experiment
- describe common mistakes that students make in physics lab reports
- provide examples of how to describe sources of error
What Are Sources of Error?
In everyday English, the words “error” and “mistake” may seem similar.
However, in physics, these two words have very different meanings:
- An error is something that affects results, which was not plausible to avoid (given the conditions of the experiment) or account for.
- A mistake is something that affects results, which should reasonably have been avoided.
We will see examples of each in the remainder of this article.
Common Incorrect Answers
Part of learning how to write a good sources of error section includes learning what not to do.
Following are some common incorrect answers that students tend to include in their sources of error section.
- Human error . The problem with this phrase is that it’s way too vague . It may be okay if the nature of the error is human in origin (provided that it’s an inherent error and not a mistake), but it’s not okay to be express the error in vague terms. Advice : Don’t write the phrase “human error” anywhere on your lab report.
- Round-off error . This problem with this is that students almost never have enough precision in their answers for round-off error to be significant. Even a cheap calculator provides at least 8 figures, whereas most first-year physics experiments yield results where only 2-3 of those digits are significant figures . You can definitely find more significant sources of error to describe instead of round-off error.
- Incorrect technique . If you used equipment incorrectly or followed the procedures incorrectly, for example, these are mistakes —they are not sources of error. A source of error is something that you could not plausibly expect to avoid.
- Incorrect calculations . If there are mistakes in your calculations, these are not sources of error. Calculation mistakes are something that students are expected to avoid. Sure, some students make mistakes, but mistakes are not sources of error.
- Accidental problem . If an accident occurred during the experiment, which could plausibly be avoided by repeating the experiment, this is not a source of error. For example, if you perform the Atwood’s machine lab and the two masses collide, it’s a mistake to keep the data. Simply redo the experiment, taking care to release the masses so that they don’t collide.
- Lab partner . Don’t blame your lab partner—at least, not in your report—because it isn’t a valid source of error.
Sources of Error: What to Look for
When you identify and describe a source of error, keep the following points in mind:
- It should sound like an inherent problem that you couldn’t plausibly avoid .
- It should be significant compared to other sources of error.
- It needs to actually affect the results . For example, when a car rolls down an inclined plane, its mass cancels out in the equation for acceleration (a = g sin θ), so it would be incorrect to cite an improperly calibrated scale as a source of error.
- You should describe the source of error as precisely as possible. Try not to sound vague .
- Unless otherwise stated by the lab manual or your instructor, you should describe the source of error in detail . In addition to identifying the source of the error, you can describe how it impacts the results, or you might suggest how the experiment might be improved (but only suggest improvement sparingly—not every time you describe a source of error), for example.
- The error should be consistent with your results. For example, if you measure gravitational acceleration in a free fall experiment to be larger than 9.81 m/s 2 , it would be inconsistent to cite air resistance as a source of error (because air resistance would cause the measured acceleration to be less than 9.81 m/s 2 , not larger).
- Try not to sound hypothetical . It’s better if it sounds like your source of error is based on observations that you made during lab. For example, saying that a scale might not be calibrated properly sounds hypothetical. If instead you say that you measured the mass to be 21.4 g on one scale, but 20.8 g on another scale, you’ve established that there is a problem with the scales (but note that this is a 3% error: if your percent error is much larger than 3%, there is a more significant source of error involved). On the other hand, if you get 20.43 g on one scale, but 20.45 g on another scale, this error is probably insignificant—not worth describing (since the percent error is below 0.1%).
- Sound scientific and objective . Avoid sounding dramatic, like “the experiment was a disaster” or “there were several sources of error.” (There might indeed be several sources of error, but usually only 1-2 are dominant and the others are relatively minor. But when you say “several sources of error,” it makes the experiment seem far worse than it probably was.)
- Demonstrate good analysis skills, applying logic and reasoning . This is what instructors and TA’s hope to read when they grade sources of error: an in-depth, well-reasoned analysis.
- Be sure to use the terminology properly. You can’t expect to earn as much credit if you get words like velocity and acceleration, or force and energy, confused in your writing.
- Follow instructions . You wouldn’t believe how many students lose points, for example, when a problem says to “describe 2 sources of error,” but a student lists 5 or only focuses on 1. Surely, if you’re in a physics class, you’re capable of counting. 🙂
Examples of Sources of Error
Here are some concrete examples of how to identify and describe sources of error.
(1) A car rolls down an incline . You measure velocity and time to determine gravitational acceleration. Your result is 9.62 m/s 2 .
A possible source of error is air resistance . This is consistent with your results: Since the accepted value of gravitational acceleration is 9.8 m/s 2 near earth’s surface, and since air resistance results in less acceleration, your result (9.62 m/s 2 ) is consistent with this source of error. If you place the car on a horizontal surface and give it a gentle push, you will see it slow down and come to rest, which shows that there is indeed a significant resistive force acting on the car.
(2) You setup Atwood’s machine with 14 g and 6 g masses. Your experimental acceleration is 3.74 m/s 2 , while your theoretical acceleration is 3.92 m/s 2 .
A possible source of error is the rotational inertia of the pulley. The pulley has a natural tendency to rotate with constant angular velocity, which must be overcome in order to accelerate the pulley. It turns out (most textbooks do this calculation in a chapter on rotation) that if the mass of the pulley is significant compared to the sum of the two masses that its rotational inertia will have a significant impact on the acceleration. In this example, the sum of the masses is 20 g (since 14 + 6 = 20). If the pulley has a mass of a few grams, this could be significant.
One way to reduce the effect of the pulley is to use larger masses. If you use 35 g and 15 g instead, the sum of the masses is 50 g and the pulley’s rotational inertia has a smaller effect. (If you do this lab, add up the masses used. Only describe this as a possible source of error if the pulley’s estimated mass seems significant compared to the sum of the masses.)
(3) You fire a steel ball using a projectile launcher . You launch the ball five times horizontally. Then you launch the ball at a 30° angle, missing your predicted target by 6.3 cm.
A possible source of error is inconsistency in the spring mechanism . If that’s all you write, however, it will sound hypothetical. If instead, you noticed variation in your five horizontal launches, which had a standard deviation (something you can calculate) of 4.8 cm, you can establish that the variation in the spring’s launches is significant compared to the distance (of 6.3 cm) by which you missed the target.
You Still Have to Think
I can’t list every possible source of error for every possible experiment—this article would go on forever.
You need to apply reasoning skills.
I have shown you what not to do.
I have shown you what to look for in a source of error.
And I have given you concrete examples for specific cases.
Be confident. You can do it. 🙂
Copyright © 2017.
Chris McMullen, Ph.D.
- Essential Physics Study Guide Workbook (3 volume series, available at the calculus or trig level)
- 100 Physics Examples (3 volume series, available at the calculus or trig level)
- The Improve Your Math Fluency series of Math Workbooks
Click here to visit my Amazon author page.
Share this:
Leave a comment cancel reply.

- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
- Wolfram|One
- Mathematica
- Wolfram|Alpha Notebook Edition
- Wolfram|Alpha Pro
- Mobile Apps
- Finance Platform
- System Modeler
- Wolfram Player
- Wolfram Engine
- WolframScript
- Wolfram Workbench
- Volume & Site Licensing
- Enterprise Private Cloud
- Application Server
- View all...
- Technical Consulting
- Corporate Consulting
- For Customers
- Online Store
- Product Registration
- Product Downloads
- Service Plans Benefits
- User Portal
- Your Account
- Support FAQ
- Customer Service
- Contact Support
- Wolfram Language Documentation
- Wolfram Language Introductory Book
- Get Started with Wolfram
- Fast Introduction for Programmers
- Fast Introduction for Math Students
- Webinars & Training
- Summer Programs
- Public Resources
- Wolfram|Alpha
- Demonstrations Project
- Resource System
- Connected Devices Project
- Wolfram Data Drop
- Wolfram + Raspberry Pi
- Wolfram Science
- Computer-Based Math
- Computational Thinking
- About Wolfram
- Wolfram Community
- Wolfram Blog
- Legal & Privacy Policy
- WolframAlpha.com
- WolframCloud.com

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
6 Sources of Experimental Error
When performing an experiment nothing can be measured with perfect accuracy. Experiments have to be designed with care to minimize potential sources of error that could skew the results away from the “true” value. Possible reasons for unexpected results might be:
- Human error (e.g. errors when following the procedure, errors in preparing solutions).
- Small sample size or differences due to biological variation
- Results were too variable to draw clear conclusions
The following Tables 1 and 2 cover common types of experimental error and other factors that might impact experimental results and how to minimize errors with experimental design.
Types of Experimental Errors and How to Minimize them with Experimental Design and Statistics
Other Types of Factors that Influence Experimental Design
Results and Discussion Writing Workshop Part 1 Copyright © by Melissa Bodner. All Rights Reserved.
Share This Book

- Previous Article
- Next Article
Critical Thinking: Error Sources and Energy in a Conservation of Momentum Lab
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Reprints and Permissions
- Cite Icon Cite
- Search Site
Robert D. Rosen; Critical Thinking: Error Sources and Energy in a Conservation of Momentum Lab. Phys. Teach. 1 April 2008; 46 (4): 235–236. https://doi.org/10.1119/1.2895675
Download citation file:
- Ris (Zotero)
- Reference Manager
Our school has a very small budget for equipment. So finding a gem like a conservation of momentum lab requiring only basic materials ( TPT , October 2005) 1 adds a great deal to my physics course. In this lab, a “shooter” nickel makes a non-head-on collision with a stationary “target” nickel. Students measure the distance each nickel slides after the collision and the angles shown in the diagram. The paper by James Hunt 1 explains how these data can be used to verify conservation of momentum in the direction perpendicular to the shooter's initial velocity. This paper suggests some possible sources of error and explains how the angles can be used to study energy relationships in the collision.
Citing articles via
Related content.
- Online ISSN 1943-4928
- Print ISSN 0031-921X
- For Researchers
- For Librarians
- For Advertisers
- Our Publishing Partners
- Physics Today
- Conference Proceedings
- Special Topics
pubs.aip.org
- Privacy Policy
- Terms of Use
Connect with AIP Publishing
This feature is available to subscribers only.
Sign In or Create an Account

IMAGES
VIDEO
COMMENTS
Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report.
This uncertainty will be present in any digital measuring device. There will be a similar uncertainty if you are reading the value from an analog device such as a pressure gauge or a d'Arsonval voltmeter. The uncertainty will then involve your estimate of the precision with which you can read the device.
experimental errors. Do not list all possible sources of errors there. Your goal is to identify only those significant for that experiment! For example, if the lab table is not perfectly leveled, then for the collision experiments (M6 - Impulse and Momentum) when the track is supposed to be horizontal, results will have a large, significant ...
The authors provide a metrology-led perspective on best practice for the electrochemical characterisation of materials for electrochemical energy technologies. Such electrochemical experiments are ...
• Understand how uncertainty is an integral part of any lab experiment Introduction There is no such thing as a perfect measurement. All measurements have errors and uncertainties, no matter how hard we might try to minimize them. Understanding possible errors is an important issue in any experimental science. The conclusions we
Error, reproducibility and uncertainty in experiments for electrochemical energy technologies. The authors provide a metrology-led perspective on best practice for the electrochemical characterisation of materials for electrochemical energy technologies. Such electrochemical experiments are highly sensitive, and their results are, in practice ...
Generally, laboratory calculations reflect the precision of a measurement, rather than limiting it (or directly affecting the accuracy). However some particular points can be sources of uncertainty. Use of physical constants can limit your accuracy or precision if you use a rounded version (e.g. 3.00×108 3.00 × 10 8 m/s instead of 299 792 458 ...
Addressing random errors often requires a repetitive approach. Since the errors are not consistent across trials, repeating the experiment multiple times and then finding a statistical mean of the results can help to mitigate the effect of random errors.
or. dy − dx. - These errors are much smaller. • In general if different errors are not correlated, are independent, the way to combine them is. dz =. dx2 + dy2. • This is true for random and bias errors. THE CASE OF Z = X - Y. • Suppose Z = X - Y is a number much smaller than X or Y.
uncertainties of an instrument, are statistical errors. There is an extensive mathematical literature dealing with statistical errors, and most of the rest of this note will be concerned with them. Systematic errors arise from problems in the design of an experiment. They are not random, and tend to affect all measurements in some well-defined way.
When listing "Sources of Error" in your lab write-up: - Give SPECIFIC examples of sources of error, and describe their probable effect on your results. Some examples that relate to specific labs: o The ruler scale is limited to measure to 1 mm o The effects of friction between the ticker tape and the recording timer were not accounted for
Apr 30, 2011. Conservation Conservation of energy Energy Lab. In summary, some errors that may have occurred in the lab include measurement inaccuracies, such as rounding errors and potential issues with the measuring device, as well as other factors such as air flow in the room. These errors could affect the accuracy of the location of the ...
Systematic Errors: faults or flaws in the investigation design or procedure that shift all measurements in a systematic way so that in the course of repeated measurements the measurement value is constantly displaced in the same way. Systematic errors can be eliminated with careful experimental design and techniques. .
In addition to identifying the source of the error, you can describe how it impacts the results, or you might suggest how the experiment might be improved (but only suggest improvement sparingly—not every time you describe a source of error), for example.
How to avoid these types of errors: Encourage students to undertake experimental inquiries carefully and to the best of their abilities. Having a clearly defined plan and well laid out work area for inquiries will help minimize accidents. Students helping to water a plant (Source: FatCamera via iStockphoto).
4. The best precision possible for a given experiment is always limited by the apparatus. Polarization measurements in high-energy physics require tens of thousands of person-hours and cost hundreds of thousand of dollars to perform, and a good measurement is within a factor of two.
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
In the diagram, note the tick marks of the meter stick and where the object falls. The end of the object falls between the tick marks. That means when we measure the length of the
As a member, you'll also get unlimited access to over 88,000 lessons in math, English, science, history, and more. Plus, get practice tests, quizzes, and personalized coaching to help you succeed.
AAPT members receive access to The Physics Teacher and the American Journal of Physics as a member benefit. To learn more about this member benefit and becoming an AAPT member, visit the Joining AAPT page.