An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

A novel experimental hypoxia chamber for cell culture
Ruoxiang wang, fengshuo jin.
- Author information
- Article notes
- Copyright and License information
Address correspondence to: Dr. Hua Zhong, Rutgers Cancer Institute of New Jersey, Department of Pathology and Laboratory Medicine, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ 08903, USA. E-mail: [email protected]
Received 2013 Nov 26; Accepted 2013 Dec 28; Collection date 2014.
Tissue hypoxia is a common pathophysiological process. Since 1990s, numerous studies have focused on investigating cellular adaptation to experimental hypoxia. A modular incubator chamber made of solid materials has frequently been used in the experiments that require hypoxic conditions. Here, we introduce a novel and inflatable chamber for hypoxia experiments. In experiments detecting hypoxia-induced accumulation of hypoxia-inducible factor 1α (HIF-1α) and hypoxia-induced expression of HIF-1-regulated genes, the new chamber yielded reproducible and comparable results as the modular incubator chamber did. The new chamber did not create inner chamber pressure during its use. Other properties of the new chamber were low-cost, easy to use, and leakage-free. Moreover, the size of the new chamber was adjustable, and the smaller one could be placed onto an inverted microscope for real-time studies. The successful examples of real-time studies included the real-time recording of GFP-HIF-1α fusion nuclear translocation and endothelial cell tubular formation.
Keywords: Cell culture, hypoxia, hypoxia chamber, hypoxia-inducible factor 1
Introduction
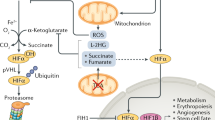
Oxygen is essential for life, and an adequate oxygen tension in internal milieu is maintained by various cellular and molecular mechanisms. Both mammalian and non-mammalian cells can sense oxygen depletion and adapt to this new environment through a series of distinctive biological processes. Among these, the activation of hypoxia-inducible factor 1 (HIF-1) is considered to be the most critical event that triggers hypoxia adaptation [ 1 ]. HIF-1 is a heterodimeric transcription factor composed of α and β subunits [ 2 ]. The α subunit of HIF-1 (HIF-1α) is sensitive to oxygen tension and dominates HIF-1 transcriptional activity, whereas HIF-1β is a constitutively expressed subunit. Under normoxia, oxygen controls HIF-1α turnover by a post-translational modification process involving hydroxylation, ubiquitination and 26S proteasomal degradation [ 1 , 3 , 4 ]. Conversely under hypoxia, the rate of HIF-1α turnover is inhibited, resulting in an increased rate of the protein accumulation, nuclear translocation, and remarkably enhanced transcriptional activity [ 1 ].
Recent studies on cellular adaptation to hypoxia have been focusing more on regulating HIF-1α in cultured cells. A reliable experimental device that can create and maintain a hypoxic environment for cell culture is essential. There are several existing models for such a purpose. One is the modular incubator chamber that can be filled with low O 2 gas containing 1% O 2 , 5% CO 2 and 94% N 2 [ 2 ]. The chamber is made of solid materials in a fixed shape and size, and is the most widely used hypoxia chamber in research laboratories in the past decades. Based on our experience, one of the common defects of this chamber is leakage. Although the leakage does not frequently occur, it does disrupt experimental processes and sometimes results an uncertainty about actual inner chamber air components. Besides, the chamber creates an inner pressure if the operation is inappropriate. Another hypoxia model is a cell culture incubator by displacing O 2 with infusion of N 2 , which was supplied by an external high-pressure liquid nitrogen tank [ 5 ]. Third one is hypoxia workstation that can offer precise control of O 2 and CO 2 as well as temperature and relative humidity [ 6 , 7 ]. It is an idea workstation that provides a hypoxic environment for a long-term cell culture [ 6 ]. The second and third models are quite expensive, and they may be infeasible or inconvenient for small laboratories that do not perform hypoxia experiments on a daily basis. Hypoxia can be mimicked by treating cells or animals pharmacologically with cobalt chloride [ 8 ], a compound that may possess unknown effects in addition to induction of HIF-1α.
In the present study, we validated an alternative module for cell culture under hypoxia. It was an inflatable chamber made of transparent plastic materials. The new chamber yielded reproducible and comparable results in hypoxia-induced HIF-1α accumulation and HIF-1-regulated gene expression. Because it was inflatable, the new chamber was unlikely to create a pressurized environment during its use. It was cost-effective, easy to use, and leakage-free even during long-term experiments. More importantly, the new chamber was size-adjustable and could be used in real-time recording of the GFP-HIF-1α fusion undergoing nuclear translocation and dynamic endothelial cell tube formation under hypoxia.
Materials and methods
Cell lines, culture conditions and reagents.
All cell lines used in cell culture, except the C4-2 and ARCaP cell lines that were previously described [ 12 ], were purchased from the American Type Culture Collection (ATCC). Human prostate cancer cell lines LNCaP, C4-2, PC-3, PC3-M, DU145 and ARCaP were routinely maintained using the T-medium (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum and antibiotics (penicillin, 100 U/ml, and streptomycin, 100 μg/ml). HUV-EC-C cell line was maintained in Ham’s F12K medium with 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 0.1 mg/ml heparin, 0.03-0.05 mg/ml endothelial cell growth supplement, and 10% fetal bovine serum. HEK293 and COS-7 cell lines were maintained in Dulbecco’s modified Eagle’s medium with 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% fetal bovine serum. Cells were plated on cell culture dishes (100 mm or 60 mm) or 6-well plates at 50% confluence 24 hours before experiment. Cells were cultured either in normal conditions (37°C and 5% CO 2 equilibrated with atmospheric O 2 in a humidified incubator) that contains 21% O 2 (hereafter referred as normoxia) or in the inflatable hypoxia chamber (1% O 2 , 5% CO 2 , balanced with N 2 and humidified) that was placed in 37°C (hereafter referred as hypoxia).
Designing and assembling the inflatable chamber
Two pieces of transparent polyester barrier membranes of 4 Mil (Kapak, Minneapolis, MN) were cut into 16 × 16-inch dimensions and were used to construct the chamber that was basically formed as a pouch by using a heat impulse sealer (American International Electric, New York, NY) through sealing three sides and allowing one side free open that could be alternatively equipped with an airtight “zip-lock”. Two small holes (5-mm in diameter) were made at the two sealed corners. At one of the corners, the hole was connected with a plastic tube as an inlet port A, about 8-mm in outer diameter and 5-mm in inner diameter, by SuperGlue. The tube was used as a port for gas exchanges ( Figure 1A ). The other hole - Port B, was left open during gas filling. Each time before being used, the chamber was sterilized, which was accomplished by placing the chamber under germicidal UV irradiation for at least 2 hours. To precede a hypoxia experiment, a 16 cm × 16 cm (adjusted according to the chamber size) glass plate was placed inside the pouch as a supporting base. To maintain an environment with proper moisture similar to that of a common CO 2 incubator, a clean sponge (4 cm × 6 cm × 2 cm) soaked with autoclaved water was put inside of the chamber before the chamber was sealed. Alternatively, a tissue culture plate containing autoclaved water was placed inside the chamber ( Figure 1B ). After the cell culture containers were placed, the free open side of the chamber was sealed with the heat impulse sealer or by closing the equipped airtight “zip-lock”. The chamber was filled with low O 2 gas through the gas exchange Port A tube. The port B was controlled by a binder clip by periodically open and close. For hypoxia experiments, N 2 equilibrated gas of 1% O 2 and 5% CO 2 (Specialty Gases Southeast, Union City, GA) was used. During gas filling, the gas flow was controlled by a single-stage regulator (Fisher) at 2 psi (pounds per square inch). After the chamber was filled to 80% of its capacity, the two Ports (A & B) were clamped or completely sealed by the heat impulse sealer. Finally, the chamber was placed into the 37°C incubator for a predetermined period of time.
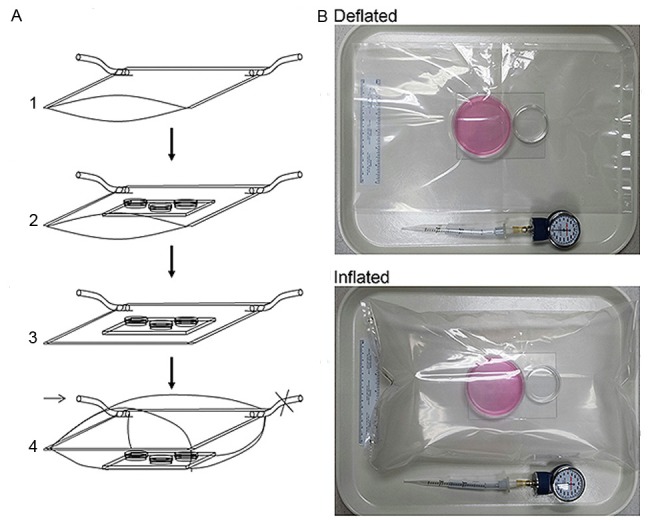
Assembly of the inflatable chamber. A. Showing an inflatable chamber in size of 16 × 16 inches. A1. An air-tight plastic pouch is prepared with two gas ports attached to the bottom corners. A2. A glass plate is placed inside the pouch as a support for culture containers. A sponge soaked with autoclaved water was placed inside of the chamber. A3. The pouch was sealed to form an insulated chamber and gently vacuumed via the gas port. A4. The chamber is filled with specified gas through the Port A (arrow) to about 80% of its maximal capacity. The gas Port B is controlled by a clamp (denoted as ×). The chamber is ready for incubation at 37°C after both ports are either closed by clamps or directly sealed at chamber corners by heat impulse sealer. B. Showing an inflatable chamber in adjusted small size that is in sealed and deflated or inflated status. Two cell culture dishes are placed on a glass plate. The large dish (60 mm) is for cell culture, and the small one (3.5 cm) contains autoclaved water. A manometer used for monitoring inner chamber atmospheric pressure after each experiment is shown.
Protein extraction and immunoblotting
The inflatable chamber was opened by incision along one side. Cells were immediately washed with ice-cold PBS and treated with the triple detergent lysis buffer freshly supplemented with protease inhibitors cocktail (Roche, Indianapolis, IN). Aliquots of 30-μg protein from whole cell lysates were fractionated onto 7.5% SDS-PAGE, and blotted onto nitrocellulose membrane (BioRad Laboratories, Hercules, CA). Monoclonal antibody to human HIF-1α (BD Transduction Laboratories, Lexington, KY) was used in 1:250 dilution. After incubation with the secondary antibody, an HRP-conjugated sheep anti-mouse IgG at 1:5000 dilution (Amersham Biosciences, Piscataway, NJ), HIF-1α was detected with an enhanced chemiluminescence kit (Amersham Biosciences). The nitrocellulose membrane was re-blotted with the antibody against β-actin.
Transient transfection and reporter gene assays
Monolayer 75% confluent cells were grown in 6-well plates and transfected in triplicate with reporter plasmids pBI-GLV6L or p2.1 (0.1 μg/well for HEK293 cells and 1 μg/well for PC-3 cells) plus control reporter pTK-RL (0.05 μg/well for HEK293 cells and 0.5 μg/well for PC-3 cells), using the Gene-Porter transfection reagent (Gene Therapy Systems, San Diego, CA). After 5 hours post transfection, the cells were allowed to recover overnight in medium containing 10% FBS. The cells were subjected to normoxia or hypoxia or for 16 hours. Plates with cells treated in hypoxia were placed inside the inflatable chamber. Luciferase activity was determined using a Dual-Luciferase Reporter System (Promega, Madison, WI) in a LUMIstar Galaxy luminometer (BMG Labtechnologies, Offenburg, Germany). Relative luciferase activity was documented by normalizing the activity of the experimental reporter ( Firefly ) to the activity of the control reporter ( Renilla ) per μg protein. Data points were statistically expressed as mean ± standard deviation.
Vascular endothelial growth factor (VEGF) ELISA
Cell culture media were used for determination of VEGF production. The DuoSet ELISA Development System for human VEGF (R & D Systems, Minneapolis, MN) was used by following with the manufacturer’s recommended protocol. The VEGF was quantified with a colorimetric assay using the substrate reagent pack (R & D Systems). Average VEGF concentration in each sample was determined with six readings. The amount of VEGF in the culture media was normalized by the amount of cellular protein, which was determined from the whole cell lysates with the BCA Protein Assay Kit (PIERCE, Rockford, IL). Data points were statistically expressed as mean ± standard deviation of two triplicate assays.
Monitoring GFP-HIF-1α translocation
Full coding sequence of the human HIF1α was cloned by RT-PCR and confirmed by DNA sequencing analysis. It was cloned in frame to the pEGFPc1 vector (Clontech), and transfected to COS-7 cells in 6-well plates using the above mentioned method. Expression of the fusion protein was confirmed by western blotting with the anti-HIF1α antibody subsequent to transfection. To study the translocation of the GFP-HIF1α fusion, the transfected cells were treated with hypoxia in the inflatable chamber which was modified in smaller size capable of 3 liters of low O 2 gas; the smaller chamber was fitted on the platform of an inverted fluorescence microscope. The hypoxia treatments started at 24 hours after transfection. At different time points after hypoxic treatment, cells inside hypoxia chamber were subjected to microscopic studies without discontinuing the hypoxic environment, followed by fluorescence imaging through the plastic chamber.
HUV-EC-C tubular formation assay
HUV-EC-C cells were seeded onto 24-well plate pre-coated with Matrigel (1:1 diluted) in 3 × 10 5 cells/well for 2 hours, followed by normoxia or hypoxia treatment. Cells subjected to hypoxia were placed into the inflatable chamber which was modified in smaller one capable of 3 liters of low O 2 gas to fit on the platform of an inverted microscope. Cell morphology was observed, and the pictures were taken at different time points between 20 minutes to 6 hours without disrupting the hypoxic environment.
Description of the inflatable hypoxia chamber
The major components of the new chamber were a transparent plastic bag and soft tubes as gas exchange ports ( Figure 1A & 1B ). The maximal volume capacity of a 16 × 16 inches size chamber was 8 liters, and the chamber contained about 6.5 liters of gas when it was filled to about 80% of its maximal volume capacity. The chamber was able to sustain the ratio of gas components for long-term cell culture experiments, such as 10 days of continuous hypoxia. After the chamber was inflated to 80% of its maximal capacity with the low O 2 gas and was incubated at 37°C, O 2 and CO 2 contents inside the chamber did not change (1% and 5%, respectively) during and after a 10-day incubation. This new hypoxia chamber was airtight, inflatable, easy to use, low-cost, and highly compliant. In addition, its size was adjustable for different purposes though it was about in 16 × 16 inches in dimension at complete deflation for most experiments. As measured by a manometer, the atmospheric pressure inside the new chamber remained at 0 mmHg by multiple tests throughout the 24 hours of incubation at 37°C.
The inflatable chamber effectively created a hypoxia environment
We next assessed the application of the inflatable chamber in hypoxia experiments. It is well known that hypoxia induces a rapid accumulation of HIF-1α, resulting the transactivation of HIF-1 target genes including the one encoding VEGF. The hypoxia-induced accumulation of HIF-1α, HIF-1-dependent transcriptional activity, and HIF-1-regulated VEGF expression were thus severed as markers to determine the effectiveness of the chamber. In a panel of human prostate cancer cell lines, HIF-1α was markedly induced after 24-h hypoxia by using the inflatable chamber ( Figure 2A ) although the basal HIF-1α levels were different among these cell lines. Similarly, significant increase in HIF-1-dependent transcriptional activity was demonstrated in PC-3 and HEK293 cells in hypoxia compared to normoxia as determined by a HIF-1/luciferase reporter that contains HRE repeats as a promoter upstream of the luciferase gene ( Figure 2B ). Additionally, secretion of VEGF in culture media was significantly increased by hypoxia in the majority of prostate cancer cell lines tested ( Figure 2C ).
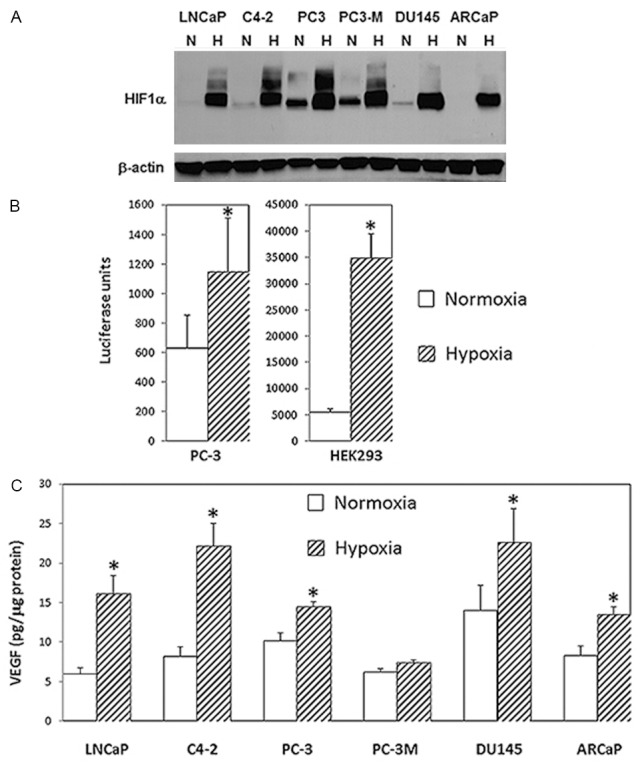
HIF-1-associated biological changes in in cells under hypoxia. A. HIF-1α as determined by western blotting in several prostate cancer cell lines in response to hypoxic treatment with the new inflatable chamber. Whole cell lysates from cells under normoxic (N) or hypoxic (H) culture conditions for 24 hours were prepared and fractionated on 7.5% SDS-PAGE before proceeding to western blotting for HIF-1α (upper panel). Western blotting for β-actin was for loading control (lower panel). B. Relative report gene activity showing in luciferase units from PC-3 and HEK293 cells in normoxia and hypoxia (new inflatable chamber). Data points were statistically expressed as mean ± standard deviation from triplicate. The (*) denotes that the luciferase units were significantly different in statistical analysis (two sample t test; A p <0.05 was considered statistically significant) between normoxia and hypoxia. C. VEGF production in culture media from prostate cancer cells in 24 hours normoxia or hypoxia (new inflatable chamber). Data in the histogram were the mean ± standard deviation from two triplicate assays (6 data points). The results generated by ELISA were normalized by the amount of cellular protein from cultured cells. The (*) denotes significantly different VEGF levels between normoxia and hypoxia in statistical analysis (two sample t test; A p <0.05 was considered statistically significant).
The inflatable chamber was applicable in hypoxia experiments for real-time studies
It is always desirable to study the cellular response to hypoxia or other specific environments on living cells at real time. Since the size of the inflatable chamber is adjustable, a smaller-sized chamber provides a good platform for such a purpose ( Figure 1B ). In a series of transient transfection assays by using a GFP-HIF1α expression plasmid, enhanced nuclear translocation of GFP-HIF-1α fusion was repeatedly demonstrated in COS7 cells treated within the inflatable hypoxia chamber in a time-dependent fashion. The enhancing signals could be observed at as early as 30 minute after placing the transfected cells under hypoxia, and the obvious enhancement in GFP-HIF-1α nuclear translocation was demonstrated at 1 hour post hypoxia treatment ( Figure 3 ). Accordingly, in cells under normoxia and at 24 hours post transfection, GFP-HIF-1α signals vaguely appeared to be more centrally located ( Figure 3A ), whereas in cells at 30 minutes under hypoxia, GFP-HIF-1α signals started to shift into nuclei ( Figure 3B ). The signals became coarse granular and were condensed within nuclei after 1 hour under hypoxia ( Figure 3C & 3D ). In contract, cells transfected control vector showed uniformed green fluorescence signals throughout cytoplasm and nuclei, showing similar pattern in cells under normoxia and under hypoxia (Data not shown).
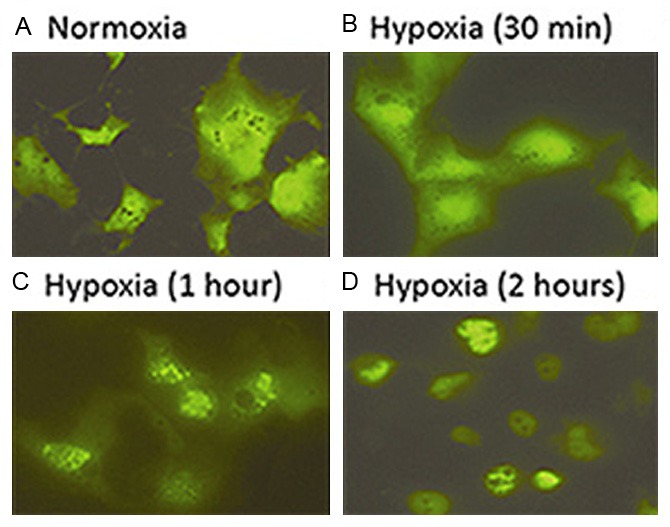
Real-time recording hypoxia-enhanced GFP-HIF-1α nuclear translocation using the small sized new chamber. Dynamic differences in cellular distribution of GFP-HIF-1α fusion were shown in normoxia (A) and different time points of hypoxia (B to D).
In another real-time study, the endothelial cell tube formation assay was performed using the inflatable chamber to create a hypoxic environment ( Figure 4 ). An inverted microscope through the small sized and transparent inflatable chamber ( Figure 1B ) was used to observe HUV-EC-C cell tubular formation that was gradually enhanced in hypoxia, and the different degree in tubular formation between normoxic and hypoxic HUV-EC-C cells became obvious at 40 minutes after different treatments ( Figure 4 ).
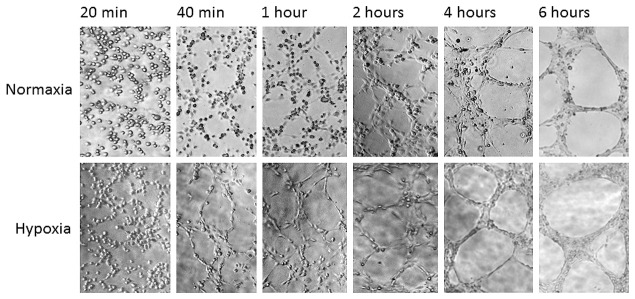
Real-time recording hypoxia-enhanced tubular formation of HUV-EC-C cells using the small sized new chamber. Cells were treated in normoxia or hypoxia (new inflatable chamber) and microscopically observed at different time points between 20 minutes to 6 hours. Photos showing dynamic cytomorphology were taken at the same locations and under the same magnification.
These results indicate that the newly designed chamber does create and maintain a hypoxic environment for cell culture, and it is an alternative tool for real-time studies of cellular response to hypoxia at both cytomorphological and molecular levels.
An ideal hypoxia chamber should be easy to use, reliable and leakage-free. An effective but low-cost chamber should be attractive to all laboratories, especially those that are new to the research field or that do not perform hypoxia experiments on a routine basis. The chamber is expected to only create an accurate low O 2 experimental setting but not to cause other environmental changes, such as an increased inner chamber pressure. According to the literature, most laboratories have utilized the modular incubator chamber in hypoxia cell culture while few laboratories have used a tissue culture incubator constantly infused with specified gas [ 5 ] or a hypoxia workstation to create a hypoxic environment [ 6 , 7 ]. Compared to the tissue culture incubator or the hypoxia workstation, the modular incubator chambers are less expensive for daily use, but it could produce inner chamber pressure or sometimes air leakage. The additional unwanted effect or air leakage is not easy to recognize and could result in unreliable data. In addition, none of the existing modules can be used for real-time study or continuous observation of cytomorphological or molecular dynamics in live cells under hypoxia. In the present study, we validated a novel and inflatable chamber for hypoxia experiments. The new chamber yields comparable results as the existing chambers usually do, and it shows additional properties that the existing chambers do not have.
The data obtained by using the new chamber were basically in agreement with those previously reported by using different hypoxia cell culture systems. For example, many previous studies have shown that HIF-1α, HIF-1 transcriptional activity and HIF-1 regulated gene expression in cultured cells under hypoxia are remarkably induced in a variety of cancer cell lines in comparison with cells under normoxia [ 1 ]. In the present study, similar expressing patterns between normoxic and hypoxic cells were reproduced by using the inflatable novel chamber ( Figure 2 ).
The newly designed hypoxia chamber possesses several important features that are relatively unique. First, the new chamber is inflatable and deflatable since it is made of soft plastic materials, so that the air in the chamber can be completely removed before the chamber is inflated with the low O 2 gas. As a result, the chamber with a precise O 2 concentration is guaranteed. Second, the chamber would never reach an abnormal pressure if the input gas volume was well-controlled during inflating. When the rigid wall based chamber is being used, a normal chamber pressure is not ensured but is often dependent on individual experience. The inner chamber pressure is in fact increased at 37°C in the rigid wall based chamber, and the inner pressure can differ among separate experiments (data not shown). An elevated chamber pressure could be a factor, directly or indirectly, altering HIF-1α and HIF-1 transcriptional activity. As demonstrated in prior studies, environmental air or hydrostatic pressure does alter gene expression profile or transcriptional activity [ 9 - 11 ]. Another important feature of the newly designed inflatable chamber is that it can be used for real-time studies of hypoxic cells under microscopy. On the contrary, other hypoxia chambers are impossible to adapt for such a purpose. In addition, the new chamber is easy to use, and even easier if it is manufactured to equip with an air-tight “zip-lock”. The new chamber is leakage-free even for a long-term experiment, which has been evidenced in several prolonged hypoxia experiments up to 10 days long (data not shown). Alternatively, the inflatable chamber could be used in studying culture cells that require a specific environmental setting other than hypoxia. The new chamber is lab-made and low-cost, and it is disposable whenever harmful contamination becomes a concern. Therefore, the inflatable chamber not only provides an accurate and worry-free experimental hypoxic setting, but also has wider applications in contrast to other hypoxia designs.
In brief summary, a new and inflatable chamber was invented for cell culture in hypoxia. It shows various advantages in comparison to those preexisting ones.
Acknowledgements
The authors thank Dr. Lorna Rodriguez (Rutgers Cancer Institute of New Jersey) for critical reading of the manuscript.
Disclosure of conflict of interest
The authors declare there is no conflict of interests regarding the publication of this article.
- 1. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Safran M, Kaelin WG Jr. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Post DE, Van Meir EG. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Edin NJ, Olsen DR, Sandvik JA, Malinen E, Pettersen EO. Low dose hyper-radiosensitivity is eliminated during exposure to cycling hypoxia but returns after reoxygenation. Int J Radiat Biol. 2012;88:311–319. doi: 10.3109/09553002.2012.646046. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Esteban MA, Maxwell PH. Manipulation of oxygen tensions for in vitro cell culture using a hypoxic workstation. Expert Rev Proteomics. 2005;2:307–314. doi: 10.1586/14789450.2.3.307. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Rodgers GM, Fisher JW, George WJ. The role of renal adenosine 3’,5’-monophosphate in the control of erythropoietin production. Am J Med. 1975;58:31–38. doi: 10.1016/0002-9343(75)90530-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Morin SM, Stotz-Potter EH, DiMicco JA. Injection of muscimol in dorsomedial hypothalamus and stress-induced Fos expression in paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1276–1284. doi: 10.1152/ajpregu.2001.280.5.R1276. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Yang P, Agapova O, Parker A, Shannon W, Pecen P, Duncan J, Salvador-Silva M, Hernandez MR. DNA microarray analysis of gene expression in human optic nerve head astrocytes in response to hydrostatic pressure. Physiol Genomics. 2004;17:157–169. doi: 10.1152/physiolgenomics.00182.2003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Wang R, Xu J, Saramäki O, Visakorpi T, Sutherland WM, Zhou J, Sen B, Lim SD, Mabjeesh N, Amin M, Dong JT, Petros JA, Nelson PS, Marshall FF, Zhau HE, Chung LWK. PrLZ, a novel prostate-specific and androgen-responsive gene of the TPD52 Family, amplified in chromosome 8q21.1 and overexpressed in human prostate cancer. Cancer Res. 2004;64:1589–1594. doi: 10.1158/0008-5472.can-03-3331. [ DOI ] [ PubMed ] [ Google Scholar ]
- PDF (1.2 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Corpus ID: 11692003
A novel experimental hypoxia chamber for cell culture.
- R. Wang , F. Jin , H. Zhong
- Published in American Journal of Cancer… 15 January 2014
- Engineering, Medicine
Figures from this paper

29 Citations
Transportable system enabling multiple irradiation studies under simultaneous hypoxia in vitro, development of a smart portable hypoxic chamber with accurate sensing, control and visualization of in vitro cell culture for replication of cancer microenvironment, an effective, low-cost method for achieving and maintaining hypoxia during cell culture studies., a facile in vitro platform to study cancer cell dormancy under hypoxic microenvironments using cocl2, engineering oxygen nanobubbles for the effective reversal of hypoxia, nucleotide depletion in hypoxia experimental models of mouse myocardial slices., evaluation of a self-regulated in vitro hypoxic system by using chemical reactions., electrochemically induced in vitro focal hypoxia in human neurons, a straightforward hypoxic cell culture method suitable for standard incubators, correlation of hypoxia and pro-senescence protein expression in green sea turtle (chelonia mydas) lung epithelial and dermal fibroblast cell culture, 12 references, manipulation of oxygen tensions for in vitro cell culture using a hypoxic workstation, a novel hypoxia-inducible factor (hif) activated oncolytic adenovirus for cancer therapy, hif hydroxylation and the mammalian oxygen-sensing pathway., low dose hyper-radiosensitivity is eliminated during exposure to cycling hypoxia but returns after reoxygenation, hypoxia-inducible factor 1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular o2 tension., hypoxia-inducible factors in physiology and medicine.
- Highly Influential
Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation
Dna microarray analysis of gene expression in human optic nerve head astrocytes in response to hydrostatic pressure., the role of renal adenosine 3',5'-monophosphate in the control of erythropoietin production., effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats, related papers.
Showing 1 through 3 of 0 Related Papers

Published in American Journal of Cancer Research 2014
R. Wang F. Jin H. Zhong
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 December 2022
Hypoxia, acidification and oxidative stress in cells cultured at large distances from an oxygen source
- Natali D’Aiuto 1 , 2 na1 ,
- Jimena Hochmann 2 , 3 na1 ,
- Magdalena Millán 2 ,
- Andrés Di Paolo 2 ,
- Ronell Bologna-Molina 4 ,
- José Sotelo Silveira 2 , 5 &
- Miguel Arocena 1 , 2
Scientific Reports volume 12 , Article number: 21699 ( 2022 ) Cite this article
5864 Accesses
1 Altmetric
Metrics details
- Biochemistry
- Cell biology
An Author Correction to this article was published on 21 June 2023
This article has been updated
Hypoxia is a condition frequently encountered by cells in tissues, whether as a normal feature of their microenvironment or subsequent to deregulated growth. Hypoxia can lead to acidification and increased oxidative stress, with profound consequences for cell physiology and tumorigenesis. Therefore, the interplay between hypoxia and oxidative stress is an important aspect for understanding the effects of hypoxic microenvironments on cells. We have used a previously developed variant of the method of coverslip-induced hypoxia to study the process of acidification in a hypoxic microenvironment and to simultaneously visualize intracellular levels of hypoxia and oxidative stress. We observed high accumulation of CO 2 in hypoxic conditions, which we show is the main contributor to acidification in our model. Also, increased levels of oxidative stress were observed in moderately hypoxic cells close to the oxygen source, where the mitochondrial membrane potential was preserved. Conversely, cells at large distances from the oxygen source showed higher levels of hypoxia, milder oxidative stress and reduced mitochondrial membrane potential. Our results contribute to characterize the interplay between reduced oxygen levels, acidification and oxidative stress in a simple in vitro setting, which can be used to model cell responses to an altered environment, such as the early tumor microenvironment.
Similar content being viewed by others
Cellular adaptation to hypoxia through hypoxia inducible factors and beyond

Long-term monitoring in a microfluidic system to study tumour spheroid response to chronic and cycling hypoxia
Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis
Introduction.
Cells have evolved sophisticated mechanisms for sensing and adapting to low levels of O 2 , which are frequently encountered in tissues, as well as during early tumor development 1 , 2 . Cellular responses to hypoxia are centered on the action of hypoxia inducible factors (HIF), requiring stabilization of the HIF-1 subunit HIF-1α, which, together with HIF-1β, form a functional HIF-1 heterodimer that activates the transcription of multiple target genes 3 . Increased levels of HIF-1α are associated with hypoxia in tissues and cells, and are commonly detected by immunostaining 4 . A more direct method for hypoxia assessment by immunostaining is the use of nitroimidazoles such as pimonidazole, which under low intracellular levels of O 2 forms adducts with cellular proteins, detectable by specific antibodies 5 . More recently, hypoxia specific fluorescent probes have become available, allowing for real time imaging of the hypoxic status of cells under different experimental conditions 6 .
The tumor microenvironment has been shown to have an acidic extracellular pH, which might constitute a selective pressure favoring the survival of more aggressive tumor cells, as well as a factor leading to decreased chemotherapy effectiveness 7 . Tumor acidification is thought to be related to lactate production by increased anaerobic glycolysis in hypoxic conditions, although CO 2 production is an alternative mechanism of acidification as well 8 . For instance, tumor cells with impaired glycolysis show an acidified microenvironment, linked to their CO 2 production 9 . Therefore, in vitro models of the tumor microenvironment that allow to determine the contributions of lactate and CO 2 production to extracellular acidification would contribute towards understanding the mechanisms of pH decrease during tumor growth.
Hypoxic conditions are also associated with higher intracellular levels of oxidative stress, particularly in the form of increased reactive oxygen species (ROS), produced mainly at the level of the mitochondrial respiratory complex III 10 . Increased ROS as a consequence of hypoxia participate in the O 2 sensing mechanism of cells 11 , but they can also have adverse consequences, particularly in the frequently hypoxic tumor microenvironment, where they contribute to increased tumor genetic instability and invasiveness 12 , 13 . Proposed mechanisms of hypoxia-induced generation of ROS include a slowed electron transport chain, which would allow partially reduced ubiquinone (ubisemiquinone) to transfer electrons to O 2 , even if O 2 levels are low 14 , 15 . Both a functional electron transport chain and the maintenance of mitochondrial membrane potential have been linked to mitochondrial generation of ROS during hypoxia 16 . In particular, mitochondrial ROS production occurs as a transient burst of superoxide (O 2 - ) generation during the first minutes of acute hypoxia, which is related to the activation of the mitochondrial Na + /Ca 2+ exchanger 17 , 18 .
Increased oxidative stress can be detected not only under low ambient O 2 but also immediately after reoxygenation, suggesting a complex interplay between varying levels of microenvironment O 2 levels and ROS production 19 . Therefore, important aspects of the study of hypoxia-induced oxidative stress are the methodologies used to recreate a hypoxic cellular environment and to evaluate intracellular hypoxiaas well as oxidative stress levels. In particular, to define more precisely the links between hypoxia and oxidative stress, methodologies devised to simultaneously assess the intracellular hypoxic and ROS status would constitute a useful contribution. Microfluidic systems have been used to expose cells to different levels of ambient O 2 while simultaneously imaging the fluorescence intensity of ROS sensitive fluorescent probes 20 . Alternatively, cell culture chambers that restrict gas exchange with the environment have been used to create cell-generated oxygen gradients, and to image hypoxic gradients and other relevant parameters, such as mitochondrial membrane potential 21 , 22 .
Recently, we have modified the method of coverslip-induced hypoxia to create simple cell culture chambers where cells can be cultured at large distances from an oxygen source, generating high levels of intracellular hypoxia that are compatible with live cell imaging 23 . In the present study, we have used this method to simultaneously visualize the hypoxic and oxidative status of cells at large distances from an oxygen source.
On the other hand, in our previous study, we used the modified method of coverslip-induced hypoxia in LNCaP cells, which is a metastatic and highly glycolytic cancer cell line, and therefore represents a cellular model of advanced cancer progression 23 . However, it would also be important to use a cellular model more representative of intermediate stages of malignant transformation, considering in particular that hypoxia in the early tumor microenvironment is a powerful selective force driving tumor progression 2 .
In the present study, we have used, as a cell model, the human keratinocyte, immortalized, non-tumorigenic HaCaT cell line, transduced with viral oncogenes E5, E6 and E7 of Human Papilloma Virus type 18 (HPV-18) oncogenes, which represents a cellular model of an intermediate stage of malignant transformation induced by high risk HPV. Previous results from our group have shown that this cell line exhibited increased levels of oxidative stress as well as a more invasive phenotype compared to the HaCaT parental cell line without viral oncogenes, which are non transformed cells 24 . In both HaCaT parental and HaCaT transduced with viral oncogenes (henceforth referred as HaCaT E5/E6/E7–18), we have observed increased oxidative stress levels when cells are close to the oxygen source, observingalso mild intracellular hypoxia levels and preservation of the mitochondrial membrane potential in this situation. In cells at large distances from an oxygen source, which are highly hypoxic, oxidative stress levels are reduced and the mitochondrial membrane potential decreases. Moreover, we have observed increased acidification, accompanied by increased lactate and CO 2 levels. Next, we have developed a simple approach to assess the contributions of lactate and CO 2 to pH decrease, showing that, in our in vitro model of the tumor microenvironment, the main contribution to acidification comes from CO 2 production.
Therefore, our results show that the modified method of coverslip-induced hypoxia can be used to recreate a hypoxic, acidified microenvironment. With this method, the contributions of lactate and CO 2 to acidification can be assessed, and in particular the oxidative and hypoxic status of cells can be visualized simultaneously, providing a method to study the contributions of different environmental stresses on cell physiology.

Intracellular hypoxia in cells at large distances from an oxygen source
HaCaT cells (both parental and E5/E6/E7–18) were cultured for 24 h under a coverslip with a central hole that serves as the only oxygen source (see Fig. 1 A for a schematic of the method). In the periphery of the cell chamber, cells developed the high levels of intracellular hypoxia that can be detected by pimonidazole, but not near the center of the chamber (Fig. 1 B), or at intermediate regions (data not shown). Therefore, we have studied intracellular hypoxia and other cellular properties (as detailed in following sections) in cells either close to the oxygen source (cells near the center of the chamber) or at a large distance from this source (cells at the periphery of the chamber). However, when instead of pimonidazole we used BioTracker™ 520 Green Hypoxia Dye to visualize intracellular hypoxia in live cells, after 24 h of coverslip treatment a moderate level of signal was observed near the center of the chamber, although the signal was much stronger in the periphery, indicating that intracellular hypoxia levels are high in the periphery but that milder levels can also be found in the central zone of the chamber (Fig. 1 D,E). O 2 levels in the culture medium are significantly reduced after 24 h of coverslip treatment (Fig. 1 C), which, taken together with the previous results, suggests that cell respiration lowers O 2 levels in the coverslip treatment condition, and therefore cells in the periphery of the chamber, far from the oxygen source, become more hypoxic than cells in the central region of the chamber, near the oxygen source.
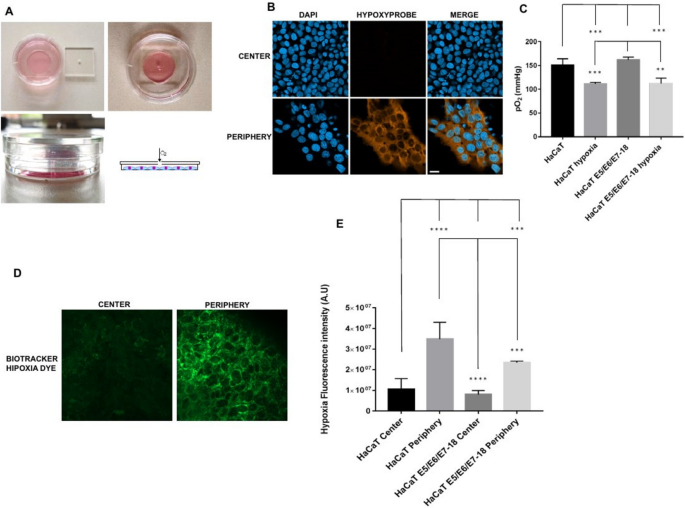
( A ) Schematics of the cell chamber used in the coverslip-induced hypoxia variant. An acrylic coverslip with a central hole is placed on top of the well of a 35 mm glass bottom dish. The central hole is the only oxygen source, and cells inside the well are at varying distances from it. ( B ) Representative images of pimonidazole adduct detection (Hypoxyprobe), shown here in HaCaT E5/E6/E7 HPV-18 cells cultured under coverslips for 24 h. Images from the central zone or the periphery of the cell chamber are shown. ( C ) Quantification of O 2 levels in HaCaT parental and HaCaT E5/E6/E7 HPV-18 cells in normoxia and with a coverslip. ( D ) BioTracker™ 520 Green Hypoxia Dye signal in control cells, or in cells under coverslips for 24 h, either in the central zone or the periphery of the cell chamber. ( E ) Quantification of hypoxia signal through BioTracker Green Hypoxia Dye in HaCaT E5/E6/E7–18 and parental HaCaT cells. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001. Scale bar: 20 μm.
Acidification, CO 2 and lactate production after coverslip-induced hypoxia
In our previous study, we observed that HaCaT E5/E6/E7–18 cells have increased expression of the glycolytic enzyme Enolase2 24 compared to parental HaCaT cells. To further characterize metabolic differences between HaCaT parental and E5/E6/E7–18 cells in normoxic conditions, we measured oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) with a Seahorse XF24 Extracellular Flux Analyzer. HaCaT E5/E6/E7–18 cells showed increased OCR and ECAR compared to HaCaT parental cell lines (Fig. 2 A,B). Slightly increased levels of lactate were also observed in the cell supernatants for HaCaT E5/E6/E7–18 cells compared to HaCaT parental cells, both in normoxia and after coverslip treatment (Fig. 2 C). Independently of these metabolic differences between HaCaT E5/E6/E7–18 cells and parental cells, both cell lines showed significantly increased levels of lactate and CO 2 in the cell supernatant after coverslip treatment (Fig. 2 D), whereas the pH of the cell supernatant decreased significantly after coverslip treatment for both cell lines (Fig. 2 E,F). These results confirm our previous observations of extracellular acidification after coverslip treatment 23 , and suggest that both lactate and CO 2 increase contribute to pH decrease.
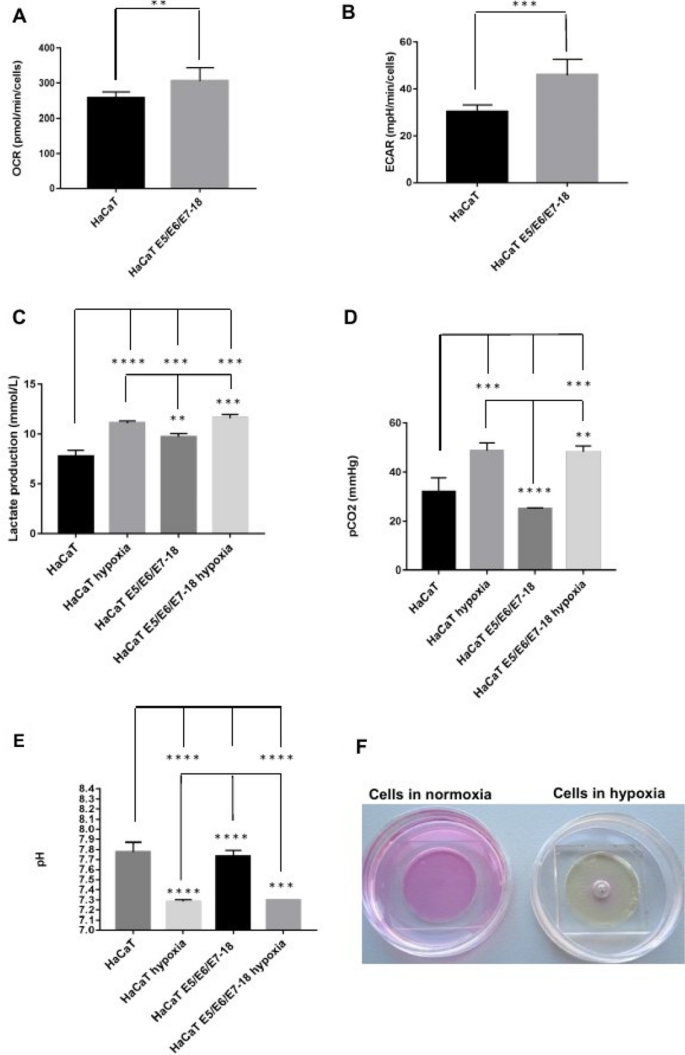
Measurement of metabolic parameters in HaCaT parental and HaCaT E5/E6/E7-18 cell lines. ( A ) Oxygen Consumption Rate (OCR) ( B ) Extracellular Acidification Rate (ECAR) ( C ) Lactate production levels measured in HaCaT parental and HaCaT E5/E6/E7–18 cells in normoxia and hypoxia conditions. ( D ) CO 2 levels measured in HaCaT parental and HaCaT E5/E6/E7-18 cells in normoxia and hypoxia conditions. ( E ) pH measurements in HaCaT parental and HaCaT E5/E6/E7–18 cells in normoxia and hypoxia. ( F ) Changes in phenol red culture medium can be seen after coverslip treatment. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001.
Contributions of CO 2 and lactate to acidification after coverslip-induced hypoxia
To assess more precisely the contributions of lactate and CO 2 to the observed pH decrease, we developed a simple approach, based on the Kassirer–Bleich approximation for the CO 2 derived bicarbonate, and on the electrical neutrality in a medium containing abundant inorganic ions such as Na + , K + , Ca 2+ and Cl − 25 . As detailed in Appendix 1 , we obtain the following equation, which relates [H + ] to [lactate] and pCO 2 :
where Δ inorganic ions is the difference in charge between the aforementioned inorganic ions. As shown in Appendix 1 , this equation fits closely the experimental pH values observed, and it allows to show in a straightforward manner that the observed increase in pCO 2 contributes much more importantly than the observed increase in lactate concentration to the observed decrease in pH. Therefore, in our model of coverslip-induced hypoxia and with the HaCaT cell lines used, the acidification we observed is related mainly to CO 2 accumulation.
Simultaneous visualization of hypoxia and oxidative stress in coverslip-induced hypoxia
Next, live cells under coverslips were incubated simultaneously with the BioTracker™ 520 Green Hypoxia Dye (498/520 nm excitation/emission) and with the ROS Detection Reagent Deep Red (640/675 nm excitation/emission). The ROS Detection Reagent signal was significantly higher near the center of the chamber (close to the oxygen source), where intracellular hypoxia was mild, than in the periphery (farther from the oxygen source), where intracellular hypoxia was high (Fig. 3 A and B).
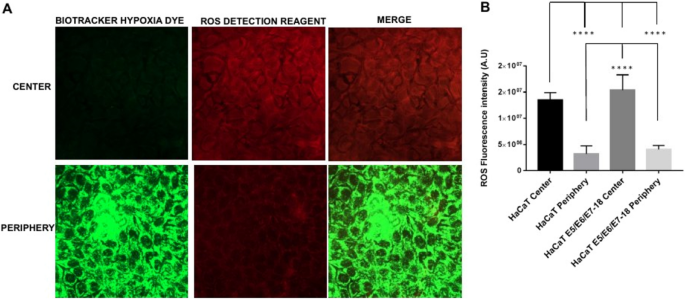
Simultaneous detection of intracellular hypoxia and ROS levels ( A ). Representative images of BioTracker™ 520 Green Hypoxia Dye, and ROS Detection Reagent Deep Red signal in cells under coverslips for 24 h, either in the central zone or the periphery of the cell chamber. ( B ) Quantification of Hypoxia and ROS intensity (mean ± SEM) in normoxia and coverslip conditions for HaCaT parental and HaCaT E5/E6/E7–18 cells.
Simultaneous visualization of hypoxia and mitochondrial membrane potential in coverslip-induced hypoxia
We then assessed the status of the mitochondrial membrane potential with the fluorescent probe Mitotracker Deep Red, that shows mitochondrial accumulation dependent upon mitochondrial membrane potential 26 , 27 , confirmed also by decreased staining intensity after treatments that reduce mitochondrial membrane potential 28 . Also, given that Mitotracker Deep Red has an excitation/emission maxima of 644/665 nm, we used it simultaneously with the Biotracker Green Hypoxia Dye. After 24 h under coverslips, cells showed significantly decreased Mitotracker Deep Red fluorescence intensity in the periphery, where the hypoxia signal was high, compared with the central region of the chamber, where the hypoxia signal was low (Fig. 4 A and B). Moreover, a 30 min treatment with the electron transport inhibitor rotenone (1 μM) caused a decrease in fluorescence intensity as well, confirming that Mitotracker Deep Red fluorescence intensity is sensitive to the mitochondrial membrane potential (Fig. 4 C). Our results therefore indicate that in the periphery of the chamber, where cells are furthest from the oxygen source and experiencing high intracellular hypoxia, mitochondrial membrane potential is significantly reduced compared to cells near the oxygen source, which experience milder levels of intracellular hypoxia.
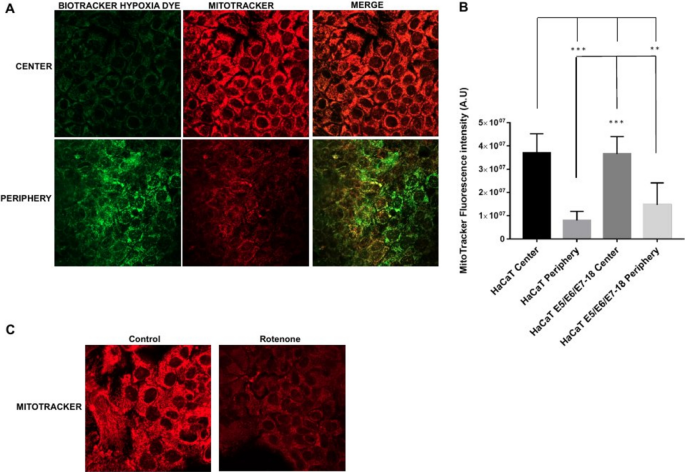
Mitochondria potential in HaCaT parental and HaCaT E5/E6/E7–18 cell lines. ( A ) Representative images of MitoTracker Deep Red FM signal in cells under coverslips for 24 h, either in the central zone or the periphery of the cell chamber. ( B ) Quantification of mitochondrial potential (mean ± SEM) in normoxia and hypoxia conditions for HaCaT parental and HaCaT E5/E6/E7–18 cells. ( C ) Representative images showing Mitotracker signal in HaCaT cells in control conditions and after 1 h rotenone (1 μM). (*) P < 0.05, (**) P < 0.01, (***) P < 0.001. Scale bar: 20 μm.
In this study, we have analyzed the acidification, hypoxia and oxidative stress generated by a modified method of coverslip-induced hypoxia that permits to culture cells at large distances from an oxygen source. We observed decreased O 2 in cell supernatants after 24 h of coverslip treatment, at which time we detected highly hypoxic cells in the periphery of the chamber, furthest from the oxygen source, both by pimonidazole staining and by using the live cell hypoxia marker BioTracker™ 520 Green Hypoxia Dye. Hypoxia detection by pimonidazole adduct formation is sensitive only to very low intracellular levels of O 2 5 , and we did not observe pimonidazole staining in cells in the central region of the chamber, near the oxygen source. However, we detected a moderate hypoxia signal in cells in this region with the BioTracker™ 520 Green Hypoxia Dye, in agreement with the manufacturers' reported properties of the Biotracker dye of detecting milder hypoxic conditions than pimonidazole. Therefore, taken together, our results can be interpreted as indicating that O 2 decreases after coverslip treatment due to cell respiration, and that cells farther from the oxygen source become as a consequence highly hypoxic, whereas cells near the oxygen source become only moderately hypoxic.
Coverslip treatment also leads to a significant decrease in the pH of the extracellular medium, while causing accumulation of both lactate and CO 2 . As shown in Appendix 1 , we derived a simple expression for [H + ] as a function of [lactate] and pCO 2 , using an approach drawing on the Kassirer–Bleich approximation for the CO 2 derived bicarbonate, and on the approach pioneered by Stewart for assessing the importance of CO 2 in the pH of body fluids, in particular the consideration of electrical neutrality in a medium containing abundant inorganic ions 25 . We obtained a very good fit between the experimental pH values and the values predicted by this expression and, in particular, using the experimental values for [lactate] and pCO 2 , it is straightforward to show that, in our experimental setting, CO 2 accumulation is the main contributor to pH decrease after coverslip-induced hypoxia. These results are in agreement with previous studies, which show that tumor acidification is related to CO 2 production by tumor cells, and that acidification can occur even in glycolysis impaired cells or cells deficient in lactate dehydrogenase, which have markedly decreased lactate production 9 , 29 . In our model, CO 2 accumulates due to the coverslip precluding gas exchange with the environment. In the microenvironment of non-vascularized or poorly vascularized tumors, CO 2 would be expected to accumulate, either by cellular respiration until oxygen is completely consumed, or by other metabolic routes, such as the pentose phosphate pathway 8 , 9 , leading to acidification. Therefore, our modified method of coverslip-induced hypoxia constitutes a simple yet useful model for analyzing the different contributions of cell metabolism towards acidification in contexts such as the tumor microenvironment.
When we visualized simultaneously hypoxia and oxidative stress, by combining the BioTracker™ 520 Green Hypoxia Dye and the ROS Detection Reagent Deep Red probe, we observed increased levels of oxidative stress in moderately hypoxic cells near the center of the chamber, whereas levels of oxidative stress in highly hypoxic cells in the periphery of the chamber were reduced. At the same time, we observed a decrease in fluorescence intensity of the MitoTracker Deep Red probe, which is sensitive to the mitochondrial membrane potential, in cells in the periphery of the chamber, where intracellular hypoxia is highest. Taken together, these results suggest that, at high levels of intracellular hypoxia, mitochondrial membrane potential decreases, leading to lower oxidative stress levels compared with cells with moderate intracellular hypoxia, in which the mitochondrial membrane potential is preserved. In agreement with these results, both a functional electron transport chain and mitochondrial membrane potential have been shown to be required for ROS production in hypoxia 16 . Moreover, the mitochondrial ROS burst that has been shown in a previous study to occur in the first minutes of hypoxia has been related to a certain degree of O 2 availability remaining inside cells at the beginning of hypoxia 17 . The cells that are close to the oxygen source in our coverslip-induced hypoxia method would be in a similar situation to the cells at the beginning of hypoxia in that study, with a certain level of O 2 remaining inside the cells (which in our cells translates as moderate intracellular hypoxia as visualized by the BioTracker™ 520 Green Hypoxia Dye). Therefore, the increased level of oxidative stress that we observed in cells close to the oxygen source is in agreement with the increased ROS production observed previously in cells at the initial stages of hypoxia 17 .
Our results also highlight the usefulness of our modified coverslip-induced hypoxia method for simultaneously visualizing levels of intracellular hypoxia and oxidative stress when studying the links between hypoxia and ROS production. In particular, the temporal dynamics of ROS production in hypoxia would be an important issue that could be analyzed with this method 17 . Also, it is important to consider cell type variabilities, as for instance similar extracellular O 2 levels can result in markedly different intracellular O 2 levels depending on the cell type, with more glycolytic cell types showing higher intracellular O 2 levels at low extracellular levels of O 2 30 . Therefore, simultaneously visualizing levels of intracellular hypoxia and oxidative stress would allow to assess whether differences in intracellular ROS levels across cell types might be related to cell type dependent levels of intracellular hypoxia under similar low O 2 culture conditions.
During tumor development, tumor cells are subjected to fluctuating O 2 levels, either in the avascular state or due to a disorganized vascular network after angiogenesis has occurred, giving rise to conditions of intermittent hypoxia, which render tumor cells more resistant to apoptotic stimuli 31 . If moderate intracellular hypoxia, rather than nearly anoxic intracellular conditions, is associated with increased intracellular ROS levels, as suggested by our results, it would be expected that tumor cells subjected to intermittent hypoxic conditions would develop milder intracellular hypoxia levels, and consequently higher intracellular ROS levels, favoring the selection of more resistant and aggressive tumor clones 12 . In addition, intermittent hypoxia could also be expected to cause increased CO 2 accumulation, due to periods of cellular respiration followed by impaired gas exchange, therefore leading to extracellular acidification, which constitutes another selective pressure towards more aggressive tumor cells 8 .
Our modified method of coverslip-induced hypoxia allows to study both the process of acidification and the interplay between intracellular hypoxia and oxidative stress as the distance from an oxygen source increases. In particular, in this study we have used HaCaT E5/E6/E7–18 cells, a recently developed cellular model of an intermediate stage of malignant transformation induced by high risk HPV, which have increased invasion capabilities and a more glycolytic metabolism compared to parental, non transduced HaCaT cells, but are still far from developing the highly glycolytic and invasive phenotypes of cell lines derived from advanced stage cancers 24 . Using this cellular model, we have been able to assess the contributions of lactate and CO 2 to extracellular acidification, and to simultaneously assess both the oxidative stress and hypoxic status of cells either close to an oxygen source or at large distances from it. Our results suggest that cells at intermediate stages of malignant transformation, when exposed to the hypoxic, avascular early tumor microenvironment, will cause extracellular acidification mainly through CO 2 accumulation, and will develop higher levels of ROS in regions closer to the capillaries from the underlying stroma, which constitute the oxygen source. Therefore, in this study we have shown that our modified method of coverslip-induced hypoxia constitutes a simple yet useful model to study how an altered microenvironment can impact processes such as tumor progression.
Materials and methods
Cell culture.
Spontaneously immortalized human keratinocyte (HaCaT) cells were purchased from Banco de células do Rio de Janeiro (BCRJ), Brazil (batch number 001071, certificate of analysis provided by the supplier) and maintained in Dulbecco’s modified eagle’s medium. (DMEM) low glucose medium (Capricorn, Ebsdorfergrund, Germany) supplemented with 10% fetal bovine serum (FBS) (Gibco, MA, USA). HaCaT cells were tested internally for mycoplasma by polymerase chain reaction (PCR). HaCaT E5/E6/E7 HPV-18 cells were obtained through co-infection with a retroviral vector carrying the MSCV-N-puro-18E5 plasmid (Addgene # 37882, MA, USA) and with a pLXSN retroviral vector that contained cloned HPV-18 E6/E7 genes gently provided by Dr. Laura Sichero. The preparation and characterization of these cell lines was detailed in Hochmann et al. 24 .
Coverslip-induced hypoxia
To culture cells at large distances from an oxygen source, we used the variant of coverslip-induced hypoxia described previously 23 . Briefly, cells were cultured in the 10 mm radius wells of glass bottom dishes (35 mm diameter, Cellvis, CA, USA), and after cells were adhered the wells were covered with square acrylic coverslips (24 mm width, 2 mm thickness) with a square hole in the middle (1 mm width). In this way, cell culture chambers are obtained where cells in the periphery of the chamber are located at 10 mm from the coverslip hole (which serves as the only oxygen source). The method is depicted in Fig. 1 A.
Hypoxia detection in fixed cells
For hypoxia detection in fixed cells, we used a hypoxia detection kit (Hypoxyprobe, MA, USA) as described previously 23 . Cells under coverslips for 22 h were incubated with the hypoxia marker pimonidazole for another 2 h at a final concentration of 200 μM in the medium. Next, cells were fixed 10 min with 4% paraformaldehyde, washed in phosphate buffered saline (PBS), permeabilized with 0.1% Triton X 100, blocked with 3% bovine serum albumin, and stained with an antibody that recognizes pimonidazole adducts conjugated to DylightTM 549 fluorophore (1:200 dilution). Nuclei were stained with 4′,6 diamidino 2 phenylindole (DAPI; Invitrogen, MA, USA). Cells were visualized with a Zeiss LSM 800 confocal microscope.
Hypoxia and ROS Detection in live cells
After 24 h of coverslip treatment, we evaluated simultaneously the levels of hypoxia and ROS in HacaT (parental and E5/E6/E7–18) live cells. At 21 h of coverslip treatment, we added both BioTrackerTM 520 Green Hypoxia Dye (Millipore, CA, USA, 5 μM final concentration) and ROS Detection Reagent Deep Red (Sigma, MO, USA, 1:1000 dilution from stock solution) through the central hole in the coverslip, or to control cells without coverslips, and after 3 h (completing 24 h of coverslip treatment) cells were visualized by confocal microscopy, and the temperature during measurements was kept at 37 °C.
Mitochondrial membrane potential detection in live cells
To detect mitochondrial membrane potential in live cells, we used the far red fluorescent dye MitoTracker Deep Red FM (Thermo Scientific, USA) that stains mitochondria in live cells and shows accumulation which is dependent upon mitochondrial membrane potential 26 , 27 .
After 21 h of coverslip treatment, MitoTracker Deep Red was added through the central hole in the coverslip or to cells without coverslip (500 nM final concentration), simultaneously with BioTrackerTM 520 Green Hypoxia Dye (5 μM final concentration) and cells were incubated for another 3 h at 37 °C, and then were visualized by confocal microscopy.
Measurements of O 2 , CO 2 , lactate and pH
HaCaT (parental and E5/E6/E7–18) cells were incubated with or without coverslips for 24 h. After this period, cells supernatants were collected and analyzed immediately in a ABL800Flex radiometer to obtain values of pH, O 2 , lactate and CO 2 levels as well as levels of important extracellular ions (see Appendix 1 ).
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) measurements
OCR and ECAR were measured in HaCaT (parental and E5/E6/E7–18) cell using a Seahorse XF24 Extracellular Flux Analyzer (Agilent, USA). Seahorse XF Wave Software was used to analyze the data.
Statistical analysis
Statistical analysis and graphical presentation were conducted using GraphPad Prism version 8.0.1 (244) software (GraphPad Software Inc., San Diego, CA, USA). All experiments were performed in triplicate and data were presented as the mean ± standard deviation (SD). Data were analyzed by One-Way unpaired ANOVA followed by Tukey’s HSD post-hoc test with the exception of data retrieved from Seahorse Analyzer, which was analyzed using Student`s Test in order to compare only two means.
Data availability
All data associated with this study are present in the paper or the Supplementary Materials.
Change history
21 june 2023.
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-36156-7
Semenza, G. L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. Mech. Dis. 9 , 47–71 (2014).
Article CAS Google Scholar
Gillies, R. J. & Gatenby, R. A. Hypoxia and adaptive landscapes in the evolution of carcinogenesis. Cancer Metastasis Rev. 26 , 311–317 (2007).
Article CAS PubMed Google Scholar
Semenza, G. L. Hypoxia-inducible factors: Coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 36 , 252–259 (2017).
Vukovic, V. et al. Hypoxia-inducible factor-1α is an intrinsic marker for hypoxia in cervical cancer xenografts. Cancer Res. 61 , 7394–7398 (2001).
CAS PubMed Google Scholar
Raleigh, J. A., Chou, S.-C., Arteel, G. E. & Horsman, M. R. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumors. Radiat. Res. 151 , 580 (1999).
Article ADS CAS PubMed Google Scholar
Piao, W. et al. Development of azo-based fluorescent probes to detect different levels of hypoxia. Angew. Chemie Int. Ed. 52 , 13028–13032 (2013).
De Milito, A. & Fais, S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 1 , 779–786 (2005).
Article PubMed Google Scholar
Kato, Y. et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 13 , 89 (2013).
Article CAS PubMed PubMed Central Google Scholar
Helmlinger, G., Sckell, A., Dellian, M., Forbes, N. S. & Jain, R. K. Acid production in glycolysis-impaired tumors provides new insights into tumor. Metabolism 1 (8), 1284–1291 (2002).
Google Scholar
Guzy, R. D. et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1 , 401–408 (2005).
Lee, P., Chandel, N. S. & Simon, M. C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21 , 268–283 (2020).
Gillies, R. J., Verduzco, D. & Gatenby, R. A. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer 12 , 487–493 (2012).
Shimojo, Y. et al. Attenuation of reactive oxygen species by antioxidants suppresses hypoxia-induced epithelial–mesenchymal transition and metastasis of pancreatic cancer cells. Clin. Exp. Metastasis 30 , 143–154 (2013).
Guzy, R. D. & Schumacker, P. T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. In Experimental Physiology , vol. 91 807–819 (Blackwell Publishing Ltd, 2006).
Klimova, T. & Chandel, N. S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 15 , 660–666 (2008).
Martínez-Reyes, I. et al. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell 61 , 199–209 (2016).
Hernansanz-Agustín, P. et al. Acute hypoxia produces a superoxide burst in cells. Free Radic. Biol. Med. 71 , 146–156 (2014).
Hernansanz-Agustín, P. et al. Na+ controls hypoxic signalling by the mitochondrial respiratory chain. Nature 586 , 287–291 (2020).
Article ADS PubMed PubMed Central Google Scholar
Abramov, A. Y., Scorziello, A. & Duchen, M. R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 27 , 1129–1138 (2007).
Berger Fridman, I., Ugolini, G. S., Vandelinder, V., Cohen, S. & Konry, T. High throughput microfluidic system with multiple oxygen levels for the study of hypoxia in tumor spheroids. Biofabrication 13 , 035037 (2021).
Article Google Scholar
Hoh, J. H., Werbin, J. L. & Heinz, W. F. Restricted exchange microenvironments for cell culture. Biotechniques 64 , 101–109 (2018).
Gilmore, A. C. et al. An in vitro tumorigenesis model based on live-cell-generated oxygen and nutrient gradients. Commun. Biol. 4 , (2021).
Arocena, M. et al. Using a variant of coverslip hypoxia to visualize tumor cell alterations at increasing distances from an oxygen source. J. Cell. Physiol. 234 , 16671–16678 (2019).
Hochmann, J. et al. Human papillomavirus type 18 e5 oncoprotein cooperates with e6 and e7 in promoting cell viability and invasion and in modulating the cellular redox state. Mem. Inst. Oswaldo Cruz 115 , (2020).
Morgan, T. J. The Stewart approach–One clinician’s perspective. Clin. Biochem. Rev. 30 , 41–54 (2009).
PubMed PubMed Central Google Scholar
Greene, A. W. et al. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 13 , 378–385 (2012).
Townley, A. R. & Wheatley, S. P. Mitochondrial survivin reduces oxidative phosphorylation in cancer cells by inhibiting mitophagy. J. Cell Sci. 133 , 575 (2020).
Mot, A. I., Liddell, J. R., White, A. R. & Crouch, P. J. Circumventing the Crabtree effect: A method to induce lactate consumption and increase oxidative phosphorylation in cell culture. Int. J. Biochem. Cell Biol. 79 , 128–138 (2016).
Yamagata, M., Hasuda, K., Stamato, T. & Tannock, I. F. The contribution of lactic acid to acidification of tumours: Studies of variant cells lacking lactate dehydrogenase. Br. J. Cancer 77 , 1726–1731 (1998).
Potter, M., Badder, L., Hoade, Y., Johnston, I. G. & Morten, K. J. Monitoring intracellular oxygen concentration: Implications for hypoxia studies and real-time oxygen monitoring. In Advances in Experimental Medicine and Biology vol. 876 257–263 (Springer New York LLC, 2016).
Martinive, P. et al. Preconditioning of the tumor vasculature and tumor cells by intermittent hypoxia: Implications for anticancer therapies. Cancer Res. 66 , 11736–11744 (2006).
Download references
Acknowledgements
This work was funded by Comisión Sectorial de Investigación Científica (CSIC), Programa de Desarrollo de las Ciencias Básicas (PEDECIBA) and Agencia Nacional de Investigación e Innovación (ANII). We are grateful to Ines Marmisolle (Centro de Investigaciones Biomédicas, CEINBIO, Facultad de Medicina, UdelaR) for her assistance in mitochondrial membrane potential experiments. We are also grateful to Valeria Valez (Centro de Investigaciones Biomédicas, CEINBIO, Facultad de Medicina, UdelaR) and Martín Angulo (Departamento de Fisiopatología, Hospital de Clínicas, Facultad de Medicina, UdelaR) for assistance with the Seahorse XF24 Extracellular Flux Analyzer and the ABL800Flex radiometer, respectively.
Author information
These authors contributed equally: Natali D’Aiuto and Jimena Hochmann.
Authors and Affiliations
Cátedra de Bioquímica y Biofísica, Facultad de Odontología, Universidad de la República, Montevideo, Uruguay
Natali D’Aiuto & Miguel Arocena
Departamento de Genómica, Instituto de Investigaciones Biológicas Clemente Estable, Montevideo, Uruguay
Natali D’Aiuto, Jimena Hochmann, Magdalena Millán, Andrés Di Paolo, José Sotelo Silveira & Miguel Arocena
Departamento de Fisiología, Facultad de Medicina, Universidad de la República, Montevideo, Uruguay
Jimena Hochmann
Departamento de Patología Molecular, Facultad de Odontología, Universidad de la República, Montevideo, Uruguay
Ronell Bologna-Molina
Sección Biología Celular, Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay
José Sotelo Silveira
You can also search for this author in PubMed Google Scholar
Contributions
N.D., J.H., M.M. and M.A. performed experiments. A.D.P., R.B.M. and J.S.S. assisted with data acquisition and analysis. M.A. developed the approach to assess pH dependence on CO 2 and lactate. N.D., J.H. and M.A. wrote the paper.
Corresponding authors
Correspondence to Jimena Hochmann or Miguel Arocena .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Acknowledgements section in the original version of this Article was incomplete. It now reads: “This work was funded by Comisión Sectorial de Investigación Científica (CSIC), Programa de Desarrollo de las Ciencias Básicas (PEDECIBA) and Agencia Nacional de Investigación e Innovación (ANII). We are grateful to Ines Marmisolle (Centro de Investigaciones Biomédicas, CEINBIO, Facultad de Medicina, UdelaR) for her assistance in mitochondrial membrane potential experiments. We are also grateful to Valeria Valez (Centro de Investigaciones Biomédicas, CEINBIO, Facultad de Medicina, UdelaR) and Martín Angulo (Departamento de Fisiopatología, Hospital de Clínicas, Facultad de Medicina, UdelaR) for assistance with the Seahorse XF24 Extracellular Flux Analyzer and the ABL800Flex radiometer, respectively.”
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
D’Aiuto, N., Hochmann, J., Millán, M. et al. Hypoxia, acidification and oxidative stress in cells cultured at large distances from an oxygen source. Sci Rep 12 , 21699 (2022). https://doi.org/10.1038/s41598-022-26205-y
Download citation
Received : 15 August 2022
Accepted : 12 December 2022
Published : 15 December 2022
DOI : https://doi.org/10.1038/s41598-022-26205-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

IMAGES
VIDEO
COMMENTS
In experiments detecting hypoxia-induced accumulation of hypoxia-inducible factor 1α (HIF-1α) and hypoxia-induced expression of HIF-1-regulated genes, the new chamber yielded reproducible and comparable results as the modular incubator chamber did.
In experiments detecting hypoxia-induced accumulation of hypoxia-inducible factor 1α (HIF-1α) and hypoxia-induced expression of HIF-1-regulated genes, the new chamber yielded reproducible and comparable results as the modular incubator chamber did.
In experiments detecting hypoxia-induced accumulation of hypoxia-inducible factor 1α (HIF-1α) and hypoxia-induced expression of HIF-1-regulated genes, the new chamber yielded reproducible and comparable results as the modular incubator chamber did. The new chamber did not create inner chamber pressure during its use.
Here, we describe simplified protocols for stabilizing cellular hypoxia-inducible factor-1α (HIF-1α) in cell culture using either a hypoxia chamber or CoCl 2. In addition, we also provide a detailed methodology to confirm hypoxia induction by the assessment of protein levels of HIF-1α, which accumulates in response to hypoxic conditions.
A novel and inflatable chamber for hypoxia experiments that yielded reproducible and comparable results as the modular incubator chamber did and included real-time recording of GFP-HIF-1α fusion nuclear translocation and endothelial cell tubular formation.
Recently, we have modified the method of coverslip-induced hypoxia to create simple cell culture chambers where cells can be cultured at large distances from an oxygen source, generating...